Manufacturing of biosimilars is more challenging than the traditional small molecule generics. Some of the reasons are:
- Investments (including operating costs) associated with manufacturing of biosimilars along with the risk of failure for biosimilars are significantly higher than that for small molecule generics. This results in a relatively smaller discount for biosimilars compared to small molecule generics.
- Minor changes in manufacturing process can cause significant changes in efficacy or immunogenicity.
- Biosimilars are larger and more complex molecules to manufacture.
The dawn of the ‘biosimilar age’, however, despite these manufacturing challenges is driven by a reduction in production costs. This cost-reduction has been possible over the past decade due to the standardisation of the manufacturing process of proteins, particularly antibiotics, with increased capacity and improvements in conventional purification technologies paving the way [1].
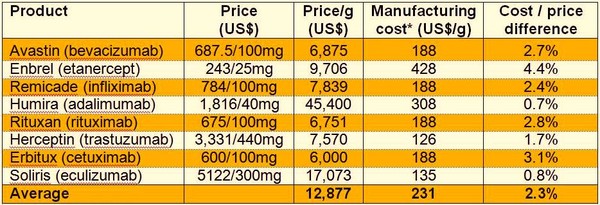
According to a Bernstein Analysis, the resulting difference between manufacturing cost and price is estimated to be on average around 2.3%, with some products having up to 4.4% difference, see Table 1. Thus, resulting in a viable product from a profit viewpoint [2].
Table 1: Difference between cost of manufacture and price
*Assuming 2g/L yield
Note: IP licensing is excluded from cost calculation; objective of analysis is to show the market from perspective of subsequent entrant.
Source: Bernstein Research
The technology to produce biosimilars is now commonplace, coupled with high operating profits and requiring limited upfront investment. This combination, being so lucrative, in turn attracts additional competition.
This competition is expected to come from ‘biobetters’ primarily developed by innovator companies and from ‘biosimilars’ developed primarily by generic companies.
Related articles
Timing of the launch of biosimilars in Europe
How cheap will biosimilars need to be
The market for biosimilars
Biosimilar regulatory issues
Development of biosimilars
Challenges ahead for biosimilar development
EGA meeting London 2011: biosimilars competitiveness in the EU
References
1. Kelley B. Industrialization of mAb production technology: The bioprocessing industry at a crossroads. mAbs. 2009;1(5):443-52.
2. Gal R. Biosimilar development is progressing, but bigger challenges are ahead. 9th EGA International Symposium on Biosimilar Medicines; 2011 Apr 14; London, UK.








 0
0











Post your comment