Non‐Biological Complex Drugs/Research
|
Posted 19/01/2018
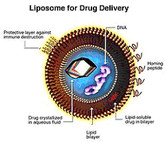
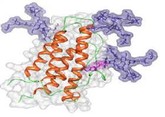
The advent of biological medicines has significantly transformed the landscapes of many disease spaces and improved the lives of millions around the world. However, the structural complexity and sensitivity of such products result in a high price tag, adding to already financially strained healthcare systems. As these and other expensive complex drugs lose market exclusivity, stakeholders eagerly await the arrival of lower cost alternatives, such as biosimilars and follow-on non-biological complex drugs (NBCDs). Nevertheless, stakeholders remain uncertain about key issues which have resulted in heterogeneous reimbursement policies and varying levels of biosimilar uptake and differences in the approval processes for follow-on NBCDs between different markets.