Up until 2002, Brazil had no specific guidance for biological products. In 2002 guidelines for biological products were published (RDC 80/2002), which had to be followed by both originator biologicals and ‘follow-on biological products’.
In 2010, Brazil introduced regulations to specifically address and establish specific pathways to license follow-on biological products using a comparability pathway. The Brazilian regulations (RDC 55/2010) are based on different regulations and guidelines from around the world, including the WHO Similar Biological Product Guidelines. They follow the same scientific principles as the WHO guidelines but contain some differences due to the specific needs of Brazil.
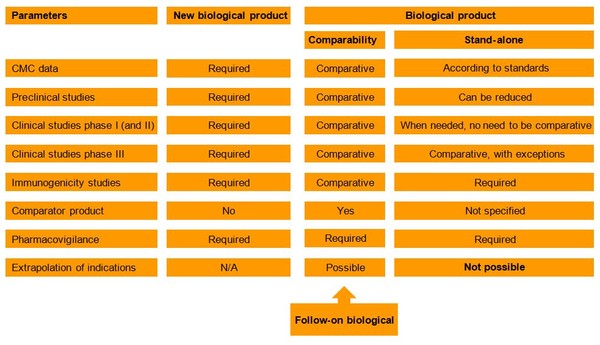
According to Resolution–RDC 55/2010 two pathways exist for non-originator biologicals – a comparability pathway and a stand-alone pathway. The two pathways have different requirements for the amount of data required for the registration dossier, see Figure 1.
Figure 1: Regulatory pathways for registration of biological products by ANVISA
ANVISA: Agência Nacional de Vigilância Sanitária; CMC: chemistry, manufacturing and controls; N/A: not applicable.
Stand-alone pathway
The stand-alone pathway is used in instances where a non-originator biological cannot be compared to the originator product. With this pathway the applicant must submit a summary of the preclinical and clinical studies performed on the non-originator biological product. The scope of the preclinical studies may be reduced and phase I/II studies can be exempt. Phase III studies are mandatory, with exceptions, and must be compared to ‘non-inferiority’, ‘equivalence’ or ‘superiority’ to the new biological product (originator biological) [1].
Comparability pathway
The comparability pathway outlines an approach in which the follow-on biological is compared to the reference product in terms of quality and available data. This pathway closely resembles that of the WHO Similar Biological Product Guidelines.
A comparative dossier containing preclinical and clinical studies is necessary to demonstrate comparability between the products. Non-clinical and clinical data can be reduced. The pathway also allows for extrapolation of therapeutic indications between the follow-on biological and the reference product.
Quality
In order to demonstrate the quality of the follow-on biological the comparability pathway demands the following during characterization of the product:
- Head-to-head comparison with the comparator biological product.
- Primary and higher-order structure, post-translational modifications, biological activity, purity and impurities, product-related substances (variants), and immunochemical properties.
- Primary structure must be identical to the comparator.
Clinical evaluation
The clinical comparability exercise requires a stepwise procedure:
- Pharmacokinetic (PK) and pharmacodynamic (PD) studies followed by pivotal clinical trials.
- Clinical efficacy studies could be required:
- The selection of a sensitive population and adequate endpoints is a critical consideration. Selection should allow the detection of possible differences between the products.
- Comparative immunogenicity study is necessary.
For the design of the studies equivalence is preferable, but non-inferiority can be used, if justified.
Extrapolation of indications
In Brazil, it is possible to receive approval for indications in which the follow-on biological has not been tested, but the following criteria must be met:
- A sensitive test model has to be used, which has to be able to detect potential differences between the biosimilar and the comparator.
- The mechanism of action and/or involved receptor(s) must be the same.
- Safety and immunogenicity have to be sufficiently characterized.
General considerations
Finally, an application for approval of a follow-on biological in Brazil has to take into consideration the following requirements:
- The pharmaceutical dosage form and the route of administration should be the same as those of the comparator
- For licensing of a biosimilar it is mandatory to present a risk management plan.
Editor’s comment
It should be noted that ‘follow-on biological products’ approved in Brazil might not have been authorized following as strict a regulatory process as is required for approval of biosimilars in the European Union. The EMA (European Medicines Agency) regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product.
Related articles
Regulation of follow-on biological products in Brazil
Producing follow-on biological products in Brazil
Brazil looks to follow-on biological products to contain costs
Brazil speeds up approval process for generics and biologicals
Reference
1. Spiewak B. Biosimilars in Brazil. Genetic Engineering and Biotechnology News. 1 October 2012.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment