The last few years have seen biosimilars the hot topic on the agenda of most countries. This is evidenced by the increasing number of biosimilars manufacturers as well as approvals for biosimilars around the world [1].
Biosimilar events from 2012 to 2014
Home/Reports
|
Posted 14/08/2014
 0
Post your comment
0
Post your comment
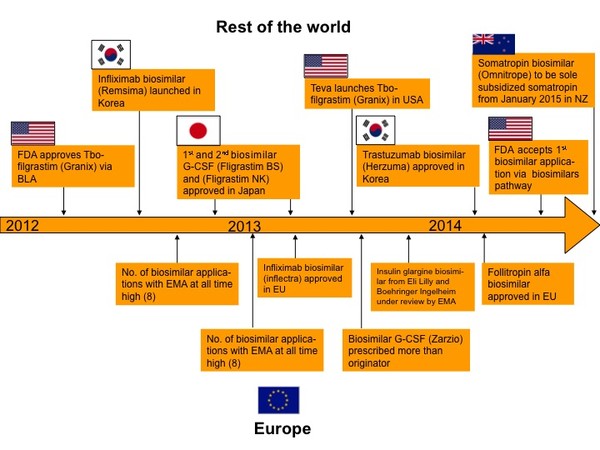
The period of 2012 to 2014 has seen biosimilars increasingly on the agenda globally, with Europe once again leading the way. Just some of the major biosimilars events that have taken place in 2012 to 2014 are shown in Figure 1.
Figure 1: Biosimilars events in the period 2012 to 2014
Teva received US Food and Drug Administration (FDA) approval for Tbo-filgrastim (Granix) on 29 August 2012. Granix was filed in the US as a Biologics License Application (BLA) since a biosimilar approval pathway had not been established at the time of submission [2]. A biosimilars pathway was established in the US via the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). FDA has recently accepted its first application via the biosimilars pathway for Sandoz’s biosimilar filgrastim product [3].
South Korea has been steaming ahead with two biosimilars approved in recent years, both from South Korean biologicals specialist Celltrion. Remsima (infliximab) was approved in July 2012 [4] and Herzuma (trastuzumab) was approved in January 2014 [5] by the Korean Ministry of Food and Drug Safety (MFDS).
Japan approved its second biosimilar granulocyte colony-stimulating factor (G-CSF), Filgrastim NK, produced by Nippon Kayaku and development partner Teva Pharmaceutical Industries (Teva) in February 2013. Japan approved its first biosimilar G-CSF (Filgrastim BS) in November 2012 [6].
Once again, Europe has been leading the way with the landmark decision on 27 June 2013 by the European Medicines Agency’s (EMA’s) Committee for Medicinal Products for Human Use (CHMP) to recommend approval of the infllximab biosimilars Inflectra and Remsima, proving that the biosimilar concept can be successfully applied to such complex molecules as monoclonal antibodies [7].
In January 2014, EMA’s CHMP also recommended the granting of a marketing authorization for biosimilar infertility treatment Bemfola (follitropin alfa) [8].
Editor’s comment
If you would like to receive a high-resolution copy* of Figure 1, please send us an email.
*For profit organizations subjected to a fee
Related articles
Biosimilars approved in South Korea
Biosimilars approved in Europe
References
1. Sheppard A. Biological/biotechnological and biosimilars market: the global outlook with special focus on Europe. 12th EGA International Biosimilar Medicines Conference; 3-4 April 2014; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Teva launches new biologicals in Europe and US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/Teva-launches-new-biologicals-in-Europe-and-US
3. GaBI Online - Generics and Biosimilars Initiative. FDA accepts biosimilar filgrastim application [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/FDA-accepts-biosimilar-filgrastim-application
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilar monoclonal antibody approved in Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-monoclonal-antibody-approved-in-Korea
5. GaBI Online - Generics and Biosimilars Initiative. Biosimilar trastuzumab approved in Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-trastuzumab-approved-in-Korea
6. GaBI Online - Generics and Biosimilars Initiative. Japan approves second biosimilar G-CSF [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/Japan-approves-second-biosimilar-G-CSF
7. GaBI Online - Generics and Biosimilars Initiative. EC approves first monoclonal antibody biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/EC-approves-first-monoclonal-antibody-biosimilar
8. GaBI Online - Generics and Biosimilars Initiative. EMA approves follitropin alfa biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Aug 14]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-follitropin-alfa-biosimilar
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment