Results from a phase III trial of biosimilar infliximab have proven the equivalence of South Korean biotechnology company Celltrion’s biosimilar (CT-P13) and the reference product – Johnson & Johnson’s rheumatoid arthritis blockbuster Remicade (infliximab) in terms of safety and efficacy in patients with active rheumatoid arthritis [1].
Biosimilar infliximab equivalence proven in phase III trial
Biosimilars/Research
|
Posted 06/07/2012
 0
Post your comment
0
Post your comment
The results of the phase III trial were presented at the Annual Congress of the European League Against Rheumatism (EULAR) held in Berlin, Germany on 6-9 June 2012.
Phase III trial
For the phase III trial 606 patients with active rheumatoid arthritis were enrolled regardless of whether they had received previous treatment with disease-modifying anti-rheumatic drugs including methotrexate or not. The patients were randomised 1:1 to receive either CT-P13 or infliximab (3 mg/kg, 2-hour intravenous infusion per dose) plus methotrexate and folic acid. The treatment period consisted of a dose-loading phase (weeks 0, 2, and 6) and a maintenance phase (weeks 14, 22, and 30).
Efficacy
At week 30, the proportion of patients achieving 20% improvement in American College of rheumatology (ACR) criteria ACR20 (see Table 1) were 60.9% for CT-P13 versus 58.6% for infliximab (2% difference; 95% CI -6% to 10%) in the intent-to-treat population, and 73.4% versus 69.7% (4% difference; 95% CI: -4% to 12%) in the per-protocol population. The exact binomial test with 95% confidence intervals (CIs) for ACR20 within a margin of ±15% was used to define equivalence between the 2 treatments.
Outcomes for the secondary efficacy endpoints were also comparable. ACR50 rates in the per-protocol population were 42.3% versus 40.6% (2% difference; 95% CI -7% to 10%) and ACR70 rates were 20.2% versus 17.9% (2% difference; 95% CI -5% to 9%) in the CT-P13 and infliximab arms, respectively, all indicating equivalence in clinical efficacy at week 30.
Safety
AEs considered by the investigators to be related to study treatment were reported for 106 (35.2%) patients and 108 (35.9%) patients in the CT-P13 and infliximab arms, respectively. Related AEs due to infection were reported in 46 (15.3%) patients and 51 (16.9%) patients in the CT-P13 and infliximab arms, respectively. 15 (5%) CT-P13-treated and 17 (6%) infliximab-treated patients experienced at least 1 infusion reaction. Tuberculosis was reported in 3 patients in the CT-P13 arm and in 1 patient in the infliximab arm.
The phase III trial proved that CT-P13 and infliximab are equivalent in terms of ACR20 in patients with rheumatoid arthritis. In addition, CT-P13 was well tolerated, with an efficacy and safety profile comparable to that of infliximab up to week 30.
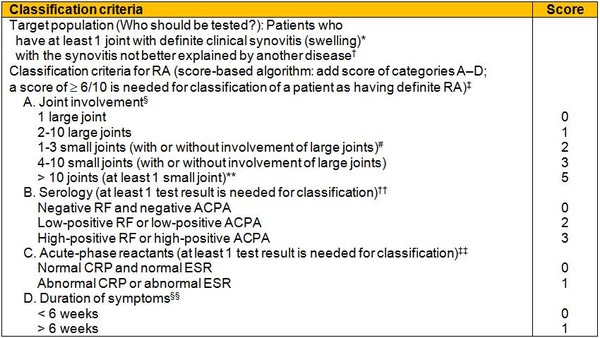
Table 1: The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis
* The criteria are aimed at classification of newly presenting patients. In addition, patients with erosive disease typical of rheumatoid arthritis (RA) with a history compatible with prior fulfilment of the 2010 criteria should be classified as having RA. Patients with longstanding disease, including those whose disease is inactive (with or without treatment) who, based on retrospectively available data, have previously fulfilled the 2010 criteria should be classified as having RA.
† Differential diagnoses vary among patients with different presentations, but may include conditions such as systemic lupus erythematosus, psoriatic arthritis, and gout. If it is unclear about the relevant differential diagnoses to consider, an expert rheumatologist should be consulted.
‡ Although patients with a score of < 6/10 are not classifiable as having RA, their status can be reassessed and the criteria might be fulfilled cumulatively over time.
§ Joint involvement refers to any swollen or tender joint on examination, which may be confirmed by imaging evidence of synovitis. Distal interphalangeal joints, first carpometacarpal joints, and first metatarsophalangeal joints are excluded from assessment. Categories of joint distribution are classified according to the location and number of involved joints, with placement into the highest category possible based on the pattern of joint involvement.
# Large joints refers to shoulders, elbows, hips, knees, and ankles.
# Small joints refers to the metacarpophalangeal joints, proximal interphalangeal joints, second through fifth metatarsophalangeal joints, thumb interphalangeal joints, and wrists.
** In this category, at least 1 of the involved joints must be a small joint; the other joints can include any combination of large and additional small joints, as well as other joints not specifically listed elsewhere, e.g. temporomandibular, acromioclavicular, sternoclavicular, etc.
†† Negative refers to IU values that are less than or equal to the upper limit of normal (ULN) for the laboratory and assay; low-positive refers to IU values that are higher than the ULN but ≤3 times the ULN for the laboratory and assay; high-positive refers to IU values that are >3 times the ULN for the laboratory and assay. Where rheumatoid factor (RF) information is only available as positive or negative, a positive result should be scored as low-positive for RF. ACPA: anti-citrullinated protein antibody.
‡‡ Normal/abnormal is determined by local laboratory standards. CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
§§ Duration of symptoms refers to patient self-report of the duration of signs or symptoms of synovitis, e.g. pain, swelling, tenderness, of joints that are clinically involved at the time of assessment, regardless of treatment status.
Source: American College of Rheumatology
Conflict of interest
Authors of the study declared that they had no conflicts of interest to report.
Editor’s comment
If you are interested in contributing a research article in a similar area to GaBI Journal, please send us your submission here.
Related articles
Phase I trial of biosimilar infliximab proves biosimilarity
Trials of biosimilar monoclonal antibody prove biosimilarity
EMA finalises biosimilar monoclonal antibody guidelines
Reference
1. Yoo D, et al. A randomized, double-blind, phase 3 study demonstrates clinical equivalence of CT‑P13 to infliximab when co-administered with methotrexate in patients with active rheumatoid arthritis. Abstract presented at: European League Against Rheumatism (EULAR) Congress. Berlin, Germany, 6–9 June 2012; abstract no. FRI0 143.
Source: American College of Rheumatology, Celltrion, EULAR
News
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment