Researchers from the Charité – Universitätsmedizin Berlin, Germany, carried out a systematic review into clinical trials for etanercept biosimilars as part of their investigation into biosimilars for the treatment of psoriasis [1].
Nast and co-authors investigated what randomized controlled clinical trials for etanercept biosimilars compared to the reference biological had been or were currently being carried out and in what indications.
The authors systematically reviewed published trials on the efficacy and safety of biosimilars and registers for planned and ongoing trials with biosimilars.
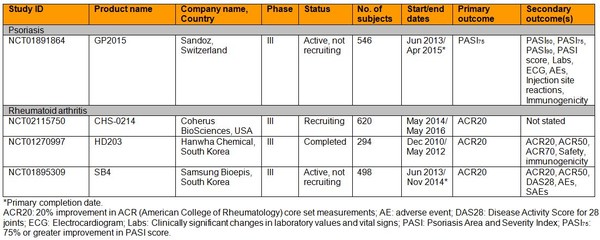
They found one registered study of an etanercept biosimilar was being carried out in psoriasis patients. Three trials were also being carried out in rheumatoid arthritis patients. No trials were identified as being carried out in ankylosing spondylitis, ulcerative colitis, Crohn’s disease or psoriatic arthritis, see Table 1.
Table 1: Clinical trials of etanercept biosimilars
For psoriasis, evidence is currently being sought for the etanercept biosimilar GP2015 in approximately 270 patients. Despite this, the authors pointed out that ‘the fact that, for instance, inflammatory bowel diseases respond to infliximab and adalimumab but not to etanercept, whereas etanercept, on the other hand, is effective in psoriasis and rheumatoid arthritis also clearly underlines the differences between the various disorders’.
The authors are therefore cautious about exchanging reference drugs with biosimilars and recommend that this ‘should only be done after careful consideration’.
Conflict of interest
The authors of the research paper [1] declared that Nast had received honoraria for CME certified educational talks that received direct or indirect sponsoring from Abbott (now AbbVie). Rosumeck and Seidenschnur declared no conflicts of interest.
Editor’s comment
Physicians, however, may not be well informed about the scientific concept underlying the principle of extrapolating* indications for biosimilars. It should be noted that infliximab cannot be used as an argument for lack of similarity of biosimilar etanercept to originator etanercept. The European Medicines Agency considers extrapolation of efficacy and safety data from one indication to another may be considered if biosimilarity to the reference product has been shown by a comprehensive comparability programme. If the relevant mechanism of action of the active substance and the target receptor(s) involved in the tested and in the extrapolated indication(s) are the same, extrapolation is usually possible [2].
*Extrapolation involves extending and applying the data from clinical studies regarding one medical condition to another medical condition.
Readers interested to learn more about building user confidence in biosimilars in Europe are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
Tell me the whole story: the role of product labelling in building user confidence in biosimilars in Europe
Readers interested in contributing a research or perspective paper to GaBI Journal – an independent, peer reviewed academic journal platform – please send us your submission here.
Related articles
Clinical trials of biosimilars for psoriasis treatment
Clinical trials for adalimumab biosimilars
Clinical trials for infliximab biosimilars
References
1. Nast A, Rosumeck S, Seidenschnur K. Biosimilars: a systematic review of published and ongoing clinical trials of antipsoriatics in chronic inflammatory diseases. J Dtsch Dermatol Ges. 2015;13(4):294-300.
2. GaBI Online - Generics and Biosimilars Initiative. Extrapolation of indications in biosimilars: infliximab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 2]. Available from: www.gabionline.net/Biosimilars/Research/Extrapolation-of-indications-in-biosimilars-infliximab
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.








 2
2









Post your comment