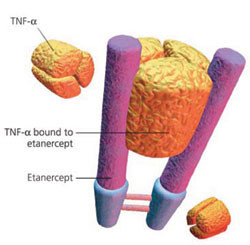
On 9 November 2015, biopharmaceutical specialists Coherus and Baxalta (a spin-off company from Baxter International) announced positive results from a phase III study of its etanercept biosimilar (CHS-0214).
The phase III, randomized, double-blind, parallel group, active-control, multicentre study was designed to compare the efficacy and safety of candidate etanercept biosimilar (CHS-0214) versus Enbrel (etanercept) in subjects with chronic plaque psoriasis. The RHAPSODY trial was planned to be completed in July 2015.
The efficacy endpoints were based on a Week 12 assessment of Psoriasis Area and Severity Index (PASI) scores. At Week 12, the primary endpoints, the mean per cent change in PASI from baseline and the proportion of subjects achieving 75% improvement in PASI from baseline, were within the pre-specified margins, which the companies claim ‘demonstrates equivalence of CHS-0214 compared to Enbrel’. The companies also claim that there were ‘no clinically meaningful differences in the safety profiles of the products’.
The study is still ongoing and will continue as planned until Week 52. According to ClinicalTrials.gov, Coherus is also carrying out a phase III trial of its etanercept biosimilar in patients with rheumatoid arthritis, as well as a long-term extension study. These confirmatory trials are intended for inclusion in global marketing applications for CHS-0214. Results for the phase III study in patients with rheumatoid arthritis are expected in the first quarter of 2016.
Coherus and Baxalta (then part of Baxter) initiated a collaboration to develop and commercialize CHS-0214 back in September 2013 [1].
South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis is the closest to having an etanercept biosimilar approved in the European Union. The company announced in January 2015 that its etanercept biosimilar candidate, SB4, had been accepted for review by the European Medicines Agency (EMA) [2].
Editor’s comment
It should be noted that data of the study presented in this article were published as a press release. These data and conclusions should be considered to be preliminary until published in a peer-reviewed journal.
Related article
Baxter and Coherus amend biosimilar etanercept collaboration
Biosimilars of etanercept
References
1. GaBI Online - Generics and Biosimilars Initiative. Baxter and Coherus to collaborate on biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Nov 27]. Available from: http://www.gabionline.net/Biosimilars/News/Baxter-and-Coherus-to-collaborate-on-biosimilars
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilar etanercept submitted for approval in EU [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Nov 27]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-etanercept-submitted-for-approval-in-EU
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.
Source: Baxalta,ClinicalTrials.gov, Coherus








 0
0









Post your comment