The US Food and Drug Administration (FDA) granted approval for two biosimilars, Formycon’s FYB202/Otulfi (ustekinumab-aauz) and Samsung Bioepis’ Soliris biosimilar, Epysqli (eculizumab-aagh), on 27 September and 22 July 2024, respectively. FYB202/Otulfi, a biosimilar referencing Johnson & Johnson’s Stelara, while Epysqli is a biosimilar referencing Alexion’s Soliris.
FDA approves biosimilars: ustekinumab Otulfi and eculizumab Epysqli
Biosimilars/News
|
Posted 29/10/2024
 0
Post your comment
0
Post your comment
Otulfi (ustekinumab-aauz)/FYB202
On 27 September 2024, FDA approved Fresenius Kabi/Formycon’s FYB202/Otulfi (ustekinumab-aauz) in both subcutaneous and intravenous formulations for the treatment of adult patients with moderate to severe plaque psoriasis, active psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Otulfi (ustekinumab-aauz) is available as 45 mg and 90 mg for injection.
The approval of Otulfi (ustekinumab-aauz) was based on a thorough evaluation of a comprehensive data package including analytical, preclinical, clinical and manufacturing data. Otulfi demonstrated comparable efficacy, safety and pharmacokinetics to the reference drug Stelara in patients with moderate to severe psoriasis vulgaris (plaque psoriasis).
In accordance with the patent settlement between Fresenius Kabi, Formycon and Johnson & Johnson, Fresenius Kabi has the right to market Otulfi in the US no later than 22 February 2025 [1].
FYB202/Otulfi (ustekinumab-aauz) was developed by Formycon, with Fresenius Kabi as their commercialization partner in Europe and the US.
Otulfi has also been approved by the European Commission on 12 September 2024 [2].
Epysqli (eculizumab-aagh)
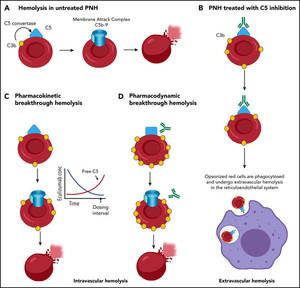
On 22 July 2024, FDA approved Samsung Bioepis’ Epysqli (eculizumab-aagh) for the treatment of patients with paroxysmal nocturnal haemoglobinuria (PNH) to reduce haemolysis, atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. EPYSQLI is not indicated for the treatment of patients with Shiga toxin E. coli related haemolytic uremic syndrome (STEC-HUS).
Epysqli (eculizumab-aagh) is available as a 300 mg/30 mL (10 mg/mL) solution for intravenous use.
The approval of Epysqli (eculizumab-aagh) was based on a totality of evidence including analytical, non-clinical and clinical data demonstrating it is highly similar to Soliris, with no clinically meaningful differences between Epysqli and Soliris in terms of safety, purity and potency: The randomized phase I, double-blind, three-arm, parallel group, single-dose study in healthy volunteers (NCT03722329) demonstrated pharmacokinetics (PK) equivalence and comparable pharmacodynamic (PD), safety, tolerability, and immunogenicity profiles between Epysqli and Soliris. Additionally, the randomized phase III, double-blind, multicentre, cross-over study in PNH patients (NCT04058158) demonstrated clinical equivalence in efficacy, safety, PK, and immunogenicity between Epysqli and Soliris.
Epysqli has also been approved by the European Commission on 26 May 2023 [3] and Korea’s Ministry of Food and Drug Safety.
FDA approves the first eculizumab biosimilar Bkemv on 28 May 2024, for the same indications as the originator and as an interchangeable biosimilar [4].
Related articles
Positive phase III results for Samsung Bioepis’ Soliris biosimilar
Alexion delays Soliris biosimilar until 2025
|
LATIN AMERICAN FORUM View the latest headline article: Los nueve fármacos biológicos más vendidos en 2023 Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Los nueve fármacos biológicos más vendidos en 2023 !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. GaBI Online - Generics and Biosimilars Initiative. Fresenius Kabi and Formycon reach agreement with J&J, Alvotech and Teva expand partnership [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Oct 29]. Available from: www.gabionline.net/pharma-news/fresenius-kabi-and-formycon-reach-agreement-with-j-j-alvotech-and-teva-expand-partnership
2. GaBI Online - Generics and Biosimilars Initiative. EC approval for three ustekinumab biosimilar: Eksunbi, Fymskina, Otulfi [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Oct 29]. Available from: www.gabionline.net/biosimilars/news/ec-approval-for-three-ustekinumab-biosimilar-eksunbi-fymskina-otulfi
3. GaBI Online - Generics and Biosimilars Initiative. EMA recommends approval of eculizumab biosimilar Epysqli [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Oct 29]. Available from: www.gabionline.net/biosimilars/news/ema-recommends-approval-of-eculizumab-biosimilar-epysqli
4. GaBI Online - Generics and Biosimilars Initiative. FDA approves first eculizumab biosimilar Bkemv for two rare diseases [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Oct 29]. Available from: www.gabionline.net/biosimilars/news/fda-approves-first-eculizumab-biosimilar-bkemv-for-two-rare-diseases
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.
Research
Reaching ESG goals in pharmaceutical development
What is the future for the US biosimilar interchangeability designation
General
Chinese biosimilars go global: growth, partnerships, and challenges
Stelara biosimilars enter US market with 85% discount in 2025
EMA recommends approval for three denosumab biosimilars: Bomyntra, Conexxence, and Rolcya

Biosimilars/News Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment