IQVIA Institute and Medicines for Europe have recently launched Country Scorecards for Biosimilar Sustainability in 12 European countries [1]. The scorecards assess the level of competition, price evolution and volume development for seven key biological molecules – adalimumab, etanercept, infliximab, insulin glargine, insulin lispro, rituximab and trastuzumab – in each country. They also present a sustainability assessment and detail positive policy elements, challenges and solutions for each country.
There are notable differences in the healthcare systems of European countries, therefore, policy scores should not be directly compared across countries. To avoid any inaccurate comparison of countries, ‘The Sustainable Market’ scorecard was developed to act as the gold-standard market to which country scorecards can compare themselves. This scorecard represents how the ideal marketplace should perform to ensure optimal market sustainability and patient-focus, and it is depicted in the tables below.
| Table 1: Contribution of biosimilars
|
|
|
| MOLECULE
|
LEVEL OF
COMPETITION
|
PRICE
EVOLUTION
|
VOLUME
DEVELOPMENT
|
|
|
1 = Low, 5 = High
|
1 = Low, 5 = High
|
1 = Low, 5 = High
|
| The most recent launches in the European market
|
An indicator of the level of competition for a molecule
|
Evolution of price level since start of biosimilar competition
|
An indicator of additional access generated since the start of biosimilar competition
|
| 7 molecules covering anti-TNFs (adalimumab, infliximab, etanercept), insulins (insulin glargine and lispro), and oncology (trastuzumab, rituximab)
|
Using a Herfindahl index to evaluate the level of competition in the market for a molecule, based on competitors’ market shares
|
Net price reduction compared to list price before biosimilar competition, collected where available
|
Increased number of treatment days (TD) per capita in Q1 2020 versus the year before biosimilar entry
|
| TNF: tumour necrosis factor.
|
|
|
| Source: IQVIA Institute for Human Data Science.
|
|
|
| Table 2: Sustainability scorecard
|
|
| POLICY AREA
|
SUSTAINABILITY MEASURE
|
SUSTAINABLE MARKET STATUS
|
| Regulatory environment and clinical guidelines
|
Time from EMA approval to first biosimilars sales
|
Instant or very short market entry after approval
|
| Treatment guidelines for biosimilar use
|
Publication of multiple guidelines on usage and protocols prior to first biosimilar entry
|
| Physician switching policies
|
Authorisation and guidance of physician-led ability to switch to a biosimilar medicine at entry of first biosimilar on the market
|
| No biologic pharmacy substitution
|
No biologic pharmacy substitution allowed
|
| Awareness and education
|
Comprehensive training / education for patient
|
Access to comprehensive and unbiased training or education prior to first biosimilar entry
|
| Comprehensive training / education for physician
|
| Incentives
|
Patient incentives to promote biosimilar use
|
Incentives in place to encourage use of most economically advantageous product upon introduction of competition
|
| Prescription quotas or financial incentives for providers that do not restrict physician choice
|
An incentive or quota that does not restrict physician choice
|
| Pricing rules and dynamics
|
Originator price not subject to mandatory price cuts
|
No forced originator price cuts by central authorities required, market forces to determine price
|
| Molecule pricing not subject to reference price
|
No reference price determined by central authorities, market forces to determine price
|
| Purchasing mechanisms
|
Length of contracts
|
12- to 24-month contracts ensure market competitiveness and avoid patients are switched often
|
| Tender timing relative to biosimilar availability
|
Tender opens upon introduction of competition
|
| Time from tender award to delivery
|
4-6 months lead time to allow necessary preparations and stock build-up
|
| Number of winners
|
Consistently award multi-winner tenders to allow of market sustainability
|
| Winner decision criteria beyond price
|
Decision based on the most economically advantageous tender offers (e.g. incorporating sustainability, price, product characteristics, continuity of supply)
|
| Source: IQVIA Institute for Human Data Science.
|
| Table 3: The sustainable market
|
| POSITIVE POLICY ELEMENTS
|
| Selected country policy elements that positively influence Biosimilar Sustainability.
|
| POLICY CHALLENGES
|
| Most-urgent country policy challenges that require action in order to achieve Biosimilar Sustainability.
|
| POTENTIAL POLICY SOLUTIONS
|
| Suggested country policy actions to address challenges and improve Biosimilar Sustainability
|
| Source: IQVIA Institute for Human Data Science.
|
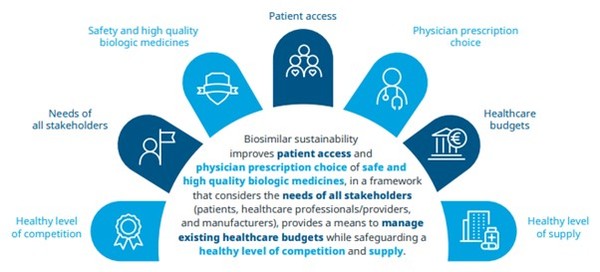
Figure 4: A multi-stakeholder definition of sustainability for the biosimilars marketplace
Successful policies balance the benefits of competition, volume uptake and price discounts. By tailoring policies to local needs, governments and authorities can reduce pressure on healthcare resources to improve access and increase the sustainability of healthcare systems.
Related articles
Sustainable biosimilar policies in Europe
Optimizing the benefits of biosimilars for society
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Scorecards show biosimilar sustainability in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Oct 2]. Available from: www.gabionline.net/Biosimilars/General/Country-scorecards-show-biosimilar-sustainability
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: IQVIA Institute for Human Data Science.








 0
0










Post your comment