First posted: 14 November 2012
Concerning the 2013 status of generics market in China, view the following related article:
The generics market in China
- The enormous population growth and the outbreak of chronic disorders and communicable diseases have driven the Chinese Government to resort to cost-effective healthcare solutions [1].
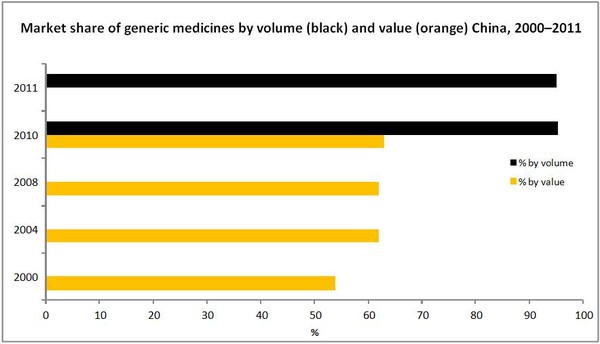
- Market shares of generic medicines in terms of value have increased from 54% in 2000 to 63% in 2010 [1], while the volume of consumption of generic medicines was 95.1% in 2011 [2].
Source: Value 2000–10 [1]; volume 2010-2011 [2]
The generic drugs market in China has gained international status and supplies finished formulations and specialty generics; it is moving beyond mass therapeutic segments like anti-infectives to niche areas like oncology, hepatic disorders and cardiovascular diseases [1].
In 2011, the Chinese pharmaceutical market was valued at US$42 billion, with generics accounting for a significant share of 63%. Branded and traditional Chinese medicine makes up the remaining 37% [1].
Given the comparative advantages of skilled workmanship, sound infrastructure and compliance with the State Food and Drug Administration over the western markets like Europe and the US, the Chinese generics market is anticipated to reach US$35 billion by 2015, making the future of generics extremely promising [1].
References
1. Frost & Sulivan. What lures the attention of global generic drug makers towards China? [monograph on the Internet]. Frost & Sulivan. 2011 Aug 12 [cited 2012 Nov 9]. Available from: www.frost.com/prod/servlet/market-insight-top.pag?Src=RSS&docid=240018520
2. Market Research.com. Generics in China 2012 [monograph on the Internet]. Market Research.com c1999-2012 [cited 2012 Nov 9]. Available from: http://www.marketresearch.com/MarketLine-v3883/Generics-China-7077415/








 0
0






Post your comment