Dr Elena Wolff-Holz gave a keynote address at Medicines for Europe’s 15th Biosimilar Medicines Conference: Biosimilar Medicines: A game changer for healthcare sustainability [1].
Dr Wolff-Holz works at the Paul Ehrlich Institut, Federal Agency for Vaccines and Biomedicines, and is Chair of the Biosimilar Medicinal Products Working Party (BMWP) of the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP).
Her address highlighted how the regulation of biosimilars in the European Union (EU) has evolved since legislation on medicines, including biologicals (Directive 2001/83/EC), was passed into law in 2001.
The European Medicines Agency first introduced overarching guidelines for biosimilars in 2005 and approved its first biosimilar Omnitropin (somatropin) in 2006 [2]. Since those initial guidelines were first published the agency has also released many product-class specific guidelines [3].
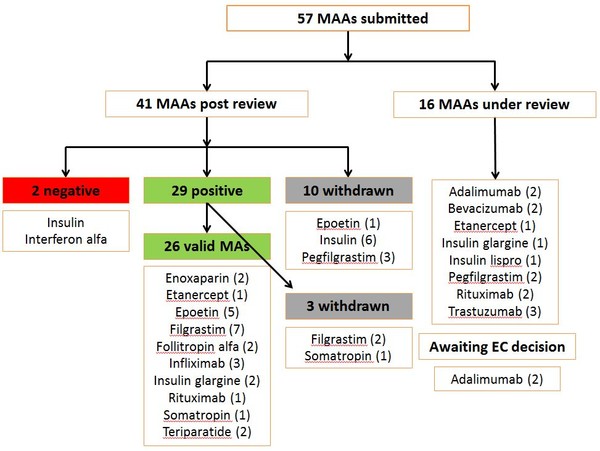
Since EMA started reviewing applications for biosimilars, and up to March 2017, the agency had received 57 marketing authorization applications (MAAs). Of these, 16 were still under review and 10 had been withdrawn (6 for insulin biosimilars, 1 for an epoetin biosimilar and 3 for pegfilgrastim biosimilars). Up to March 2017 the agency had given a positive opinion for 29 MAAs [of which 3 were later withdrawn (2 for filgrastim and 1 for somatropin)] and a negative opinion for 2 MAAs (interferon alfa and insulin). There were also 2 adalimumab biosimilars awaiting a final decision by the European Commission (EC), see Figure 1.
Figure 1: Biosimilars review by EMA (to March 2017)
EMA: European Medicines Agency; MAs: marketing authorization; MAAs: marketing authorization applications.
In summary, there are 17 distinct biosimilars (=26 products) in the EU, exist for 10 different reference biological products.
In March and August 2017, the adalimumab biosimilars Amgevita (ABP 501), Solymbic (ABP 501) and Imraldi (SB5) received approval in the EU. In June 2017, the etanercept biosimilar Erelzi (GP2015) received approval in the EU. And in June and July 2017, the rituximab biosimilars Rixathon (GP2013) and Ritemvia (CT P10) also received approval in the EU [4].
In April 2017, EMA’s Committee for Medicinal Products for Human Use (CHMP) gave a positive opinion for the rituximab biosimilar, Riximyo (GP2013) and in May 2017 for the insulin lispro biosimilar, Insulin lispro Sanofi. In September 2017, the CHMP also gave positive opinions for the adalimumab biosimilar, Cyltezo (BI 695501) and for the trastuzumab biosimilar, Ontruzant [4].
Related articles
Is a biosimilar forever or just for Christmas?
The importance of EPARs
References
1. Wolff-Holz E. Welcome Keynote Address. 15th Biosimilar Medicines Conference: Biosimilar Medicines: A game changer for healthcare sustainability; 23−24 March 2017; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars use in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Reports/Biosimilars-use-in-Europe
3. GaBI Online - Generics and Biosimilars Initiative. EU guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Guidelines/EU-guidelines-for-biosimilars
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment