Structural analysis – the starting point
Structural analysis of a biopharmaceutical product, be it a biosimilar or new drug, inevitably requires the use of a wide range of techniques and technologies. The ICH Q6B [1] guidelines for structural investigation, which are invoked by the regulatory authorities as the document detailing their expectations for structural characterization of biosimilars [2-4], state that all areas of biomolecular structure must be investigated. This requirement covers primary amino acid sequence and post-translational modifications including glycosylation (if present), through to secondary and tertiary structure and aggregation assessments, as well as an assessment and characterization of product related impurities (forms of the product that are not structurally consistent with the product specification). Structural analysis is therefore the starting point in the development of a drug product. It goes hand in hand with developing the product itself, as well as the manufacturing process, to produce the expected end product suitable for use in pharmacokinetics (PK), pharmacodynamics (PD) and clinical trials as necessary.
Structural investigations are performed at the drug development stage to examine and indeed confirm that the product has been made correctly, and also to assess the nature and levels of product related impurities prior to any clinical-based work. It is therefore understandable that analytical methods are liable to evolve as structural data are generated and interpreted, and any further specifically targeted analytical studies are carried out. To this end, there needs to be a degree of flexibility in the details of the analytical methodologies involved, since the precise conditions required for optimal data generation within a specific technique may not yet be defined.
At this point in the development of the product there is therefore no requirement for the application of specifically qualified or validated methods for analysis since the methods, product structure and therefore any product specifications or parameters are not yet defined. This does not mean to say of course that the methods used should not be demonstrated as being fi t for purpose and indeed, prior to analysis, investigations need to be performed to optimize a method for application to that particular product. This may include the demonstrated requirement for excipient removal if that is likely to interfere with analysis, e.g. high levels of sugar or amino acid excipients which would interfere with monosaccharide and amino acid analysis, respectively, or assessment of incubation times for a proteolytic digest associated with a peptide mapping study. Purification may be carried out so that both biosimilar and innovator drugs are subsequently analysed from within the same buffer system, which precludes the influence of buffer effects on results. If up-front purification is required for excipient removal, then an assessment needs to be made as to any possible impact on the active ingredient being assessed [2].
‘Fit for purpose/intended use’
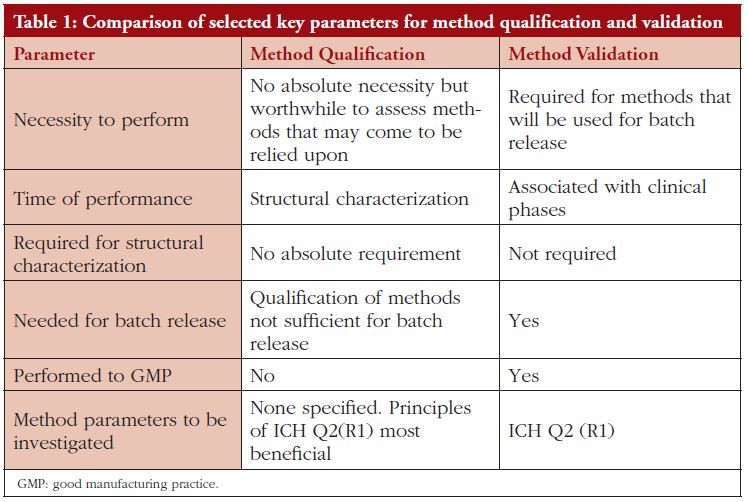
When we consider biosimilars, the requirement for the application of such a broad range of characterization techniques, with their inherent need for a degree of flexibility to suit the molecule under investigation, is recognized by the regulatory agencies as falling outside the area of ‘Quality Control’ since these procedures are used as part of product development and ultimately product definition, see Table 1 for comparison of qualification and validation features.
To this end the methods are not therefore required to be validated in accordance with ICH Q2(R1) (5) but; as stated by the US Food and Drug Administration (FDA) in their Biosimilar guidance document (Quality), methods ‘…should be scientifically sound, fi t for their intended use, and provide results that are reproducible and reliable’ [3]. The European Medicines Agency (EMA), in their Biosimilar guidance document (Quality) state ‘Methods used in the characterization studies form an integral part of the quality package and should be appropriately qualified for the purpose of comparability. If applicable, standards and reference materials, e.g. from European Pharmacopoeia (Ph. Eur.), World Health Organization (WHO), should be used for method qualification and standardization’ [2]. The UK Medicines and Healthcare products Regulatory Agency (MHRA) Biosimilars guidance document echoes this sentiment, stating ‘Analytical methods need to be sensitive, qualified and sufficiently discriminatory to detect possible differences. Robust data require the application of suitable orthogonal methods’, [4].
The idea of the suitability of methods for their analytical application, i.e. being fi t for purpose, is clearly recognized in these regulatory documents but at the same time the need for release method type controls of methodologies is also understood to be unnecessary at this characterization stage. Strength of data and the subsequent conclusions drawn for structural characterization purposes are derived from the use of orthogonal techniques where possible, a point emphasized in the guidelines.
For biosimilar analysis, qualification is useful for the determination of the expected experimental range or error of the method being employed. With this knowledge in hand, a comparison can be made between innovator and biosimilar-derived values, allowing a better assessment of similarity or otherwise between samples for the parameter in question.
Qualification of methods and instrumentation
There is no explicit mention of an expected procedure for qualification in the quoted biosimilar guidelines, and no request drawing upon any principles of the ICH Q2(R1) document on validation of analytical methods. However, when qualifying any method, the application of the principles in this document for generation of a qualified (as opposed to ‘validated’) method in a broad sense would seem wise. Use of appropriate standards to qualify a procedure, in terms of both instrumentation and methodology, can demonstrate its general applicability to a particular analysis with due regard given to whether the method is assessing a particular aspect of the sample in a qualitative or quantitative sense (through considerations of specificity, repeatability, accuracy, linearity, LOD, LOQ, intermediate precision and robustness as appropriate). The method then becomes suitable for use in characterization protocols where the same tenets hold true of the sample as for the standard used in the qualification.
This general method qualification should be repeated on a cycle of one or two years where the same process is repeated to demonstrate that the system (i.e. instrument and methodology) is still performing within the defined parameter range. Where consideration may need to be given to unique features of any biosimilar that requires specific assessment in a method application, qualification may be performed once a method has been developed specifically for that product and structural aspect. An example of this could be a method used to assess the presence of a unique or uncommon posttranslational modification.
It should be noted that the basic ‘go-to’ analytical techniques used during product characterization have usually been applied across the analyses of many molecules, including biosimilars, over time and thus have been demonstrated to have basic applicability within the area of analytical structure determination. Therefore, specific qualification of these methods may not be appropriate.
Wherever possible, appropriate checks of the method should be used during the course of sample analysis regardless of whether the method is qualified or not. These checks normally comprise of system suitability tests (SSTs) run at the start and end of an instrument analytical session. The data obtained serve to demonstrate that detection parameters, such as sensitivity and resolution, are optimal and therefore sample results are valid and data can be processed and interpreted. If there are a high number of samples to run, SSTs can also be run at regular intervals throughout the sample runs as an ongoing check that the system has not deviated from the optimal run parameters during the course of analysis. This of course can also be supplemented by interspersed repeat runs of key preparations, such as standard mixtures or blanks, to ensure a consistent and optimal level of performance across the analytical session. The SST may well have its own set of acceptance criteria that must be met, these criteria having come from a qualification of the method and thus a knowledge of how the SST will behave.
One other point to consider is the use of the term ‘qualification’ itself. Qualification does not necessarily only cover the method itself. Qualification is also performed at the time of installation of analytical instruments, in order to demonstrate performance at the expected level for a variety of defi ned parameters, e.g. sensitivity and resolution. Instrument qualification procedures cover installation, operation and performance qualifications (often described as IQ, OQ and PQ, respectively). On this basis, the instrument itself is then qualified to operate as expected and the method can then be developed with confidence in the system it is being run on.
Method qualification examples: extinction coefficient and assessments of impurities
Extinction coefficient determination
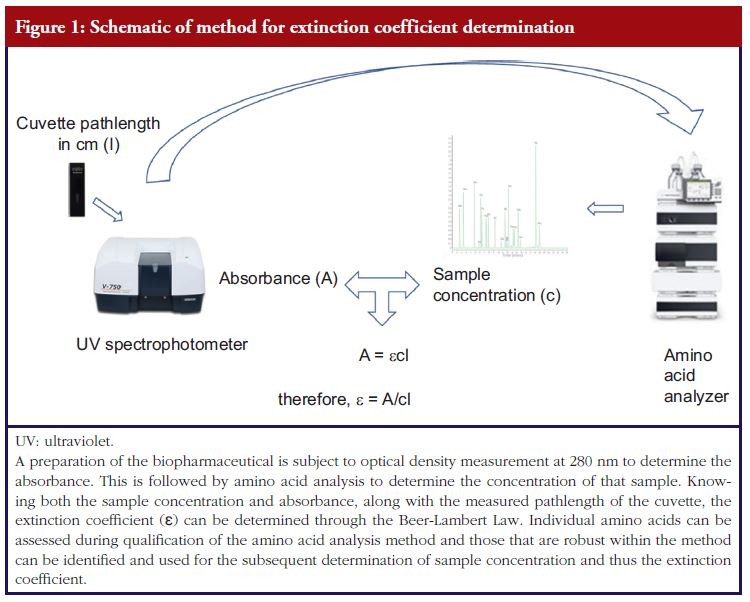
One area where method qualification is important at an early stage of product development is in determining the extinction coefficient (ε). The extinction coefficient is the measure of light absorption per unit amount of sample and is intimately related to absorbance (A), through the Beer-Lambert Law A=εcl, to the concentration of the sample (c) and pathlength (l) of the sample through which the light passes. Thus, in order to determine the concentration of a biopharmaceutical active ingredient from an absorbance measurement, one must determine the extinction coefficient.
For determination of the extinction coefficient, concentration of the protein/glycoprotein can be measured using amino acid analysis, see Figure 1, and the determined concentration assessed alongside the measured optical density and a value obtained. The significance of this value is that it can be used to measure the concentration of all prepared samples using an ultraviolet (UV) spectrophotometer for studies in subsequent biological analyses. It is critical that the amount of sample used in these studies is accurately known for dosing and safety reasons. Thus, a qualification of the methods used to determine the extinction coefficient (optical density (OD) spectroscopy and amino acid analysis) is important in demonstrating that the value obtained is coming from a reliable method and therefore can itself be analytically supported and relied upon.
Impurities
Another area that the ICH Q6B and biosimilarity guidelines highlight is that of assessing impurities present in the product. For biosimilar products it is not expected that the impurities will be the same as the innovator since the manufacturing process will not necessarily be the same. Impurities are defi ned as being either product or process related. Product related impurities are the result of differential processing of the product either during biosynthesis or subsequent purification. These are considered part of product characterization itself and will thus naturally be identified and characterized during those analyses. Process related impurities cover all aspects of the drug production process where contaminants are likely to be derived both from cellular and production sources. Removal of these impurities to the extent possible, or at least to below set limits, is critical for product quality and safety.
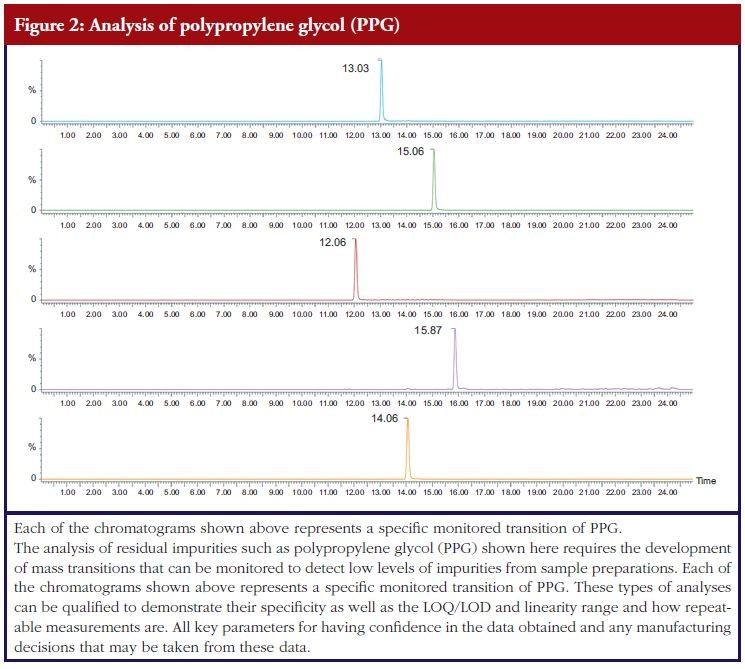
Methods that assess impurities in the characterization phase can be used in the absence of qualification, however given the importance of impurity clearance as a key consideration in the drug production process it is worth considering qualification of residual testing methods for key process-related impurities, see Figure 2.
This will allow confidence in the method and thus support for the results generated, so that meaningful changes can be made to the production process, if necessary, on the basis of reliable data. This alleviates a potential area of risk that could be very costly and time-consuming to fi x if issues with levels of impurities were discovered at a later date. These qualified methods can, if required, be subsequently validated for batch release purposes.
Methods for critical quality attributes
As mentioned above, structural analysis uses a wide variety of instrumentation and techniques to probe all aspects of biopharmaceutical/biosimilar structure. Once the structure is determined (using multiple batches of biosimilar and innovator, if the product is a biosimilar), and the manufacturing process has been tied down, batches can be manufactured for subsequent biological testing.
At this point, the methods that will be used for testing of particular key structural features, or critical quality attributes, should be considered. These methods are designed to demonstrate that the batch being tested is showing the expected structure with no anomalies or aberrations. Tests to consider will vary between different products but should provide unique information on the molecule and be responsive to a change in molecular structure such that the method should reliably detect any change in the product.
Example: Peptide mapping- MS or UV detection?
The on-line LC/ES-MS analysis used for the peptide mapping study during the characterization phase can potentially be reduced down to UV detection rather than MS detection, once structure and the manufacturing process are determined. This means that, with all other parameters, such as column and gradient conditions remaining constant, a simple fingerprint profile could be used, where the identities of the UV peaks are known from the initial MS characterization work. This method can be qualified/validated to demonstrate its applicability prior to use in batch release which would include an assessment of the sensitivity of the method to any structural changes that may occur, for example, variation in the levels of post-translational modifications. Other tests will need to be considered to monitor other aspects of molecular structure, such as intact mass analysis and glycan profiling, since peptide mapping is not a catch-all for assessment of all aspects of a molecule’s structure.
Qualification can de-risk future validation
Ultimately, appropriately selected methods used to assess the quality of a product will need to be validated and performed in a GMP environment. The decision to qualify a method in no way affects the necessity to also validate the method for the later phases of drug development, with validation of methods expected by the regulatory authorities at these stages of drug development. So what good is qualification of a method as a sort of half-way house? This comes down to the need or desire to ensure that the method is producing reliable and meaningful data for the structural aspect of the product being measured, such that the quality of the product and the process is built in from an early stage. A method, qualified through the principles of ICH Q2(R1), should be straightforward to validate since that method will already have gone through the testing phase. It will therefore stand the validation process with less risk than an untried method that has come to be relied upon but whose characteristics have not been tested rigorously.
No hard and fast rules
Method qualification is something that needs to be considered during product development based on the nature of the specific analysis being undertaken and the nature of the methodology being used. For example, qualifications of methods for residual testing and impurities, or other methods used to demonstrate the correct nature of a particular structural feature may be viewed differently compared to qualifications of structural characterization techniques using mass spectrometry. There is no hard and fast rule covering when to qualify a method or what methods should be qualified, but the qualification of selected methods early in product development can help to avoid problems with test processes that may be required for use later but which are shown to have features that preclude meeting validation criteria.
In summary, what is required at the early stage of product development, i.e. at the characterization and definition stage, is that methods are fi t for purpose and scientifically accurate such that data derived from these methods are trustworthy and sound. Thus, in biosimilar analysis, the comparative data generated will allow meaningful conclusions to be drawn regarding structural similarities and differences.
About the author: Richard obtained his PhD in glycoprotein structural characterization using mass spectrometry from Imperial College of Science, Technology and Medicine. He subsequently spent several years there as a postdoctoral research scientist working in the fi eld of glycoprotein structural characterization with emphasis on glycan elucidation. The projects he was involved in required detailed structural analysis of glycoproteins derived from animal, plant and fungal systems, very frequently expressing unusual glycosylation profiles. He moved to GlaxoSmithKline for a short time where he was head of mass spectrometry for the toxicoproteomics and safety assessment group. Richard joined M-Scan Limited (now part of SGS Life Sciences) in 2003 as a biochemist and became the Team Leader for Carbohydrate Analysis before being appointed Principal Scientist. Richard joined BioPharmaSpec in 2016 as Technical Director for Structural Analysis and is responsible for management of all aspects of carbohydrate and glycoprotein characterization at the primary structure level.
Funding sources
This paper is sponsored by BioPharmaSpec Ltd (www.biopharmaspec.com).
References
1. European Medicines Agency. ICH Topic Q6B specifications: test procedures and acceptance criteria for biotechnological/biological products. Guidance Document. August 1999 [homepage on the Internet]. [cited 2022 Mar 28]. Available from: https://www.ema.europa.eu/en/ich-q6bspecifications-test-procedures-acceptance-criteria-biotechnologicalbiological-products
2. European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). EMA/CHMP/ BWP/247713/2012. 22 May 2014 [homepage on the Internet]. [cited 2022 Mar 28]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similarbiologicalmedicinal-products-containing-biotechnologyderived-proteins-active_en-0.pdf
3. U.S. Food and Drug Administration. Development of therapeutic protein biosimilars: comparative analytical assessment and other quality-related considerations. Guidance for Industry. Draft Guidance. May 2019 [homepage on the Internet]. [cited 2022 Mar 28]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidancedocuments/development-therapeutic-proteinbiosimilars-comparative-analytical-assessmentand-other-quality
4. Government UK. Guidance on the licensing of biosimilar products [homepage on the Internet]. [cited 2022 Mar 28]. Available from: https://www.gov.uk/government/publications/guidance-on-thelicensing- of-biosimilar-products
5. European Medicines Agency. ICH Q2(R1) validation of analytical procedures: text and methodology. June 1995 [homepage on the Internet]. [cited 2022 Mar 28]. Available from: https://www.ema.europa.eu/en/documents/scientificguideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf








 0
0







Post your comment