Big savings can be made after the introduction of generics, especially when it comes to high volume categories, such as statins. Pfizer’s Lipitor (atorvastatin) was the best-selling drug of all time until it lost its patent protection at the end of June 2011 [1] and a generic version became available in the US in November 2011 [2]. However, the impact of the introduction of generics on use of Lipitor is not known. Therefore, researchers from Duke and Yale Universities analysed trends in use and expenditures associated with Lipitor after generic atorvastatin became available [3].
US adults 18 years or older in the nationally representative Medical Expenditures Panel Survey (MEPS) database from 2012 to 2014 were included in the analysis. Survey participants self-reported medication names, number of prescriptions, out-of-pocket (OOP) cost and payment source; pharmacies provided total expenditure and utilization.
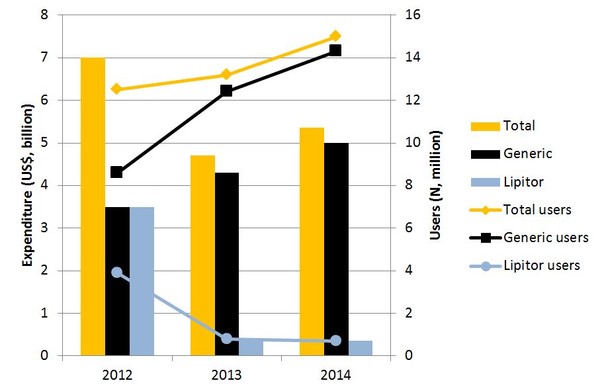
Out of the 110,789 participants included in the MEPS database between 2012 and 2014, 75,174 were eligible for the study. During this period, total atorvastatin users increased from 12.5 million to 15.0 million (20% increase) while total atorvastatin-associated expenditures decreased from US$7.0 billion to US$5.4 billion (23% decrease), see Figure 1.
Figure 1: Trends in use and cost of atorvastatin 2012–2014
Total US expenditure on Lipitor reduced from US$3.5 billion (50% of total atorvastatin cost) in 2012 to US$357 million (7% of total cost) in 2014. Excess expenditure associated with continued Lipitor use was therefore estimated to cost the US US$2.1 billion from 2012 to 2014.
The authors concluded that ‘generic availability resulted in marked increase in total atorvastatin use with reduced overall costs’. However, they noted that persistent use of the brand-name drug, Lipitor, resulted in US$2.1 billion of excessive waste in the period studied. They therefore advised that ‘swifter substitution to generic medications is necessary to substantially reduce healthcare costs’.
Related article
Lipitor generics saved UK NHS £350 million in first 12 months
Teva won’t sell generic atorvastatin
References
1. GaBI Online - Generics and Biosimilars Initiative. Can generic competition succeed at reducing cost of atorvastatin? [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 May 4]. Available from: www.gabionline.net/Generics/Research/Can-generic-competition-succeed-at-reducing-cost-of-atorvastatin
2. GaBI Online - Generics and Biosimilars Initiative. Teva gains tentative approval for generic atorvastatin [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 May 4]. Available from: www.gabionline.net/Generics/News/Teva-gains-tentative-approval-for-generic-atorvastatin
3. Warraich HJ, Salami JA, Khera R, Valero-Elizondo J, Okunrintemi V, Nasir K. Trends in use and expenditures of brand-name atorvastatin after introduction of generic atorvastatin. JAMA Intern Med. 2018 Mar 10. doi: 10.1001/jamainternmed.2018.0990. [Epub ahead of print]
4. Warraich H, Salami J, Khera R, Okunrintemi V, Nasir K. Excess national US expenditures associated with Lipitor use after introduction of generic atorvastatin: insights from the medical expenditures panel survey, 2012-2014. J Am College Cardiol. 2018;71(11) Supplement March 2018. doi:10.1016/S0735-1097(18)32305-2
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment