Introduction
Biosimilars are biopharmaceutical products intended to act as alternatives to the innovative biological products with the same mechanism of action. Biosimilars are approved after the patent expiry of an innovator product after they demonstrate same quality, efficacy and safety standards as that of the innovator biological [1]. Once the biological product is approved as ‘biosimilar’, it may be considered as ‘interchangeable’ provided clinical studies support it.
Interchangeability refers to achievement of same clinical result in any given patient in terms of quality, safety and efficacy when a biosimilar is switched or substituted with its respective innovator biological product, when compared to the use of the reference product alone. In principle, once the biosimilar product gains ‘interchangeable’ status, it can be automatically substituted for the prescribed biological product by the pharmacist without the consent of the prescribing physician [2]. However, this provision is subject to the state laws enforcing substitution legislation.
While the interchangeability between a biosimilar and its reference product is simple in theory, several processes make it difficult in practice. The guidelines or legislations for the demonstration of interchangeability are far more stringent when compared to the demonstration of biosimilarity with the reference product [3, 4]. While the biosimilar developing companies are seriously pursuing these products as an immediate alternative to high-cost branded biologicals, the initiatives and support from regulatory agencies in addressing the issues like interchangeability and substitution has been sluggish and underwhelming. The article highlights the gravity of the current legislative scenario in the US and the EU regarding the biosimilar interchangeability bills and the issues decelerating the process.
Legislative scenario in the US
The Biologics Price Competition and Innovation Act (BPCI Act) in the US has established an abbreviated approval pathway for biological products to demonstrate similar efficacy and safety with the innovator product. The federal law has differentiated the approval of products into two stages: (1) with basic similarity to the innovator product referred to as ‘biosimilar’; (2) requires additional approval status called as ‘interchangeable biosimilar’. The approval for interchangeability is rigorous to achieve and [US Food and Drug Administration] (FDA) demands the safety data to establish that no additional risk is incurred by the patient when switched between two products compared to the use of the innovator product alone [5].
The role of FDA is to approve biosimilar medicines including the grant of interchangeable status to the biosimilar once the guidelines for the regulatory approval pathway for biosimilars are in place. As of 2014, FDA is still in the process of developing guidelines for the approval of biosimilars. However, many of the states in the US have already adopted or considering the laws related to the legislation for the substitution of biologicals at the retail pharmacy level. The laws passed by the states will provide legal mechanism and requirements for the substitution of innovator biologicals with the biosimilars. The states in the US have similar substitution laws for certain generics drugs, e.g. narrow therapeutic index drug, which require physician notification before substitution. However, the law for biosimilar interchangeability is more stringent and requires record keeping also [6].
Biotechnology Industry Organization (BIO) has depicted affirmation in the FDA and its states to ensure patient safety and access to high quality, cost-effective biosimilars. The requirement for the biosimilar substitution is summed up by BIO in its 5 principles [7]:
1) Substitution should occur only when FDA has approved the biological product as ‘interchangeable’
2) The prescribing physician holds the decision-making authority to prevent the biosimilar substitution in the interest of the patient
3) The pharmacist should inform the prescribing physician in case of substitution
4) The patient or the patient’s authorized representative should be notified in case of substitution
5) The prescribing physicians and pharmacist should keep records of the substitution
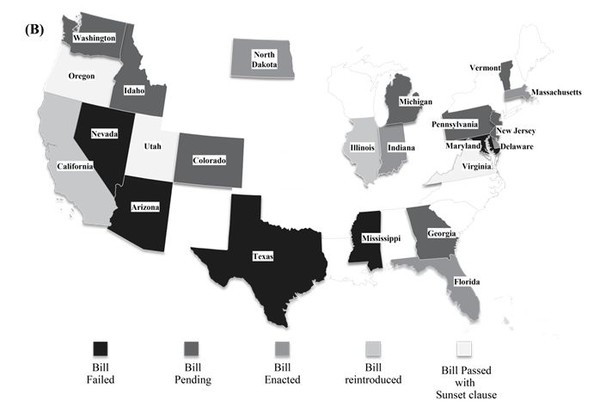
In the US, the biosimilar substitution bill was passed in eight states, namely Massachusetts [8], Florida, Virginia, Delaware [9], Indiana [10], Utah [11], North Dakota and Oregon, as of September 2014 [12]. Virginia was the first US state to pass a law regarding biosimilar substitution before the products were marketed in these regions [13]. Three states, Oregon, Utah and Virginia, have included the Sunset clause in their biosimilar substitution bill pertaining to the section on notification to physicians, which is likely to expire in 2015 in Utah and Virginia [12]. The bill is pending in seven other states, Colorado, Idaho [14], Washington [15], Georgia [16], Michigan [17], Vermont [18] and Pennsylvania. The biosimilar substitution bill was rejected in California, Nevada [19], Arizona, Mississippi [20], Illinois [21], Texas and Maryland [12]. However, California and Illinois have reintroduced the bills to approve substitution [22]. Figure 1 (B) represents bill status in the various states. All the states that accept interchangeability require that the particular drug is deemed as ‘interchangeable’ by FDA. The recent high rate of approval is consistent with the focus on cost saving since the biosimilar is expected to be significantly cheaper.
In the states, where biosimilar substitution bill is passed, pharmacists have the right to substitute a biological with a biosimilar product. However the pharmacist has to notify the physician in a given time frame which differs from state to state. For instance, prescriber should be informed within 1 day by written or electronic notice in North Dakota while the pharmacists in Indiana have to notify in any form, within 10 days as per bill passed by Indiana legislative assembly. Likewise the record retention period in electronic form for the pharmacist also varies from state to state from 2 years to a maximum of 10 years [12].
Legislative scenario in the European Union
The European Medicines Agency (EMA) was the first regulatory body to apply the concept of comparability to biosimilars. Omnitrope being the first biosimilar approved in the European Union (EU) [23], biosimilars are marketed and commercialized in the EU since 2006. However, there is still a concern among the physicians about its use due to lack of evidence on post-marketing surveillance and apprehensions on efficacy, extrapolation and interchangeability [24].
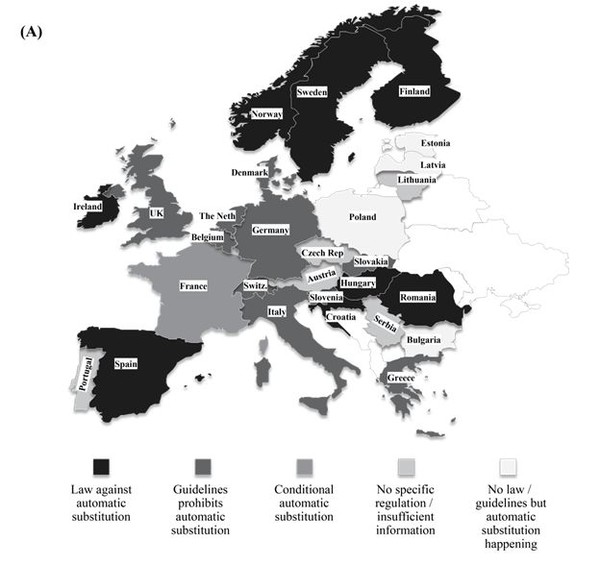
Biosimilar products are approved based on the requirements framed by EMA to show similarity in terms of efficacy and safety in comparison with the originator product. However, EMA does not have the authority to designate a biosimilar as ‘interchangeable’ unlike FDA and therefore does not evaluate biosimilar interchangeability. The decisions on the interchangeability of biosimilars and innovator products rest with the Member States in the EU. The individual countries have the authority to introduce substitution bill in their respective legislative assemblies. A representation of the status is provided in Figure 1 (A). The European Generic medicines Association (EGA) reported that more than 12 countries across the EU have introduced rules to avert automatic substitution of innovator biologicals with biosimilars [24].
France has taken the pioneer step in allowing a restricted form of biosimilar substitution in Europe and this may set the trend across Europe in the near future to generate healthcare savings. The Article 47 of the 2014 French Social Security Financing Law allows for limited pharmacy-level substitution by pharmacist when the following conditions are met [25]:
1) When the treatment is initiated for a patient
2) The biosimilar belongs to the ‘similar biologic group’ as that of the prescribed biological
3) Substitution is not prohibited by the physician (‘non substitutable’ on the prescription)
4) Inclusion of substitutable similar biologic in lists prepared by ANSM (Agence nationale de sécurité du médicament et des produits de santé) [26]
The Article 47 of the 2014 French Social Security Financing Law was effective from 1 January 2014. According to the provisions in the law, the pharmacist in France can substitute a biosimilar with a prescribed biological product only if the physician has not prohibited the substitution. However, the new rules cannot be implemented until the relevant decrees regarding the precise set of conditions required for biosimilar interchangeability have been adopted [27].
The role of the physician is crucial to identify the patients who are prescribed with the biological for the first time and specify the same in the prescription to identify naïve patients. The pharmacist has to maintain records and notify the physician and patient about the biosimilar dispensed in case of substitution [26].
In rest of the EU5 countries (except France), as of date, there is no authorization for the substitution of biosimilars with innovator biologicals from different manufacturers. In Germany, the pharmacist may substitute a biosimilar as part of the obligatory generic substitution referred to as ‘Aut-idem-Regelung’. Nevertheless, there is no authorized legislation in the country to substitute biologicals from different manufacturers [28]. In the UK, Italy and Spain, automatic substitution in community pharmacies is not allowed. The Italian Medicines Agency (AIFA), however, has recently stated that physicians in Italy should consider prescribing biosimilars to treat naïve patients, if it leads to considerable healthcare savings. The Netherlands and Austria have a more neutral approach. In The Netherlands, substitution is allowed with other biosimilars but never with originator molecule. In case of Austria, substitution is promoted on naïve patients and this discretion usually lie with the prescribing physician. Although these countries have accepted the significance of biosimilars, the legislation for interchangeability is far from visibility.
Figure 1: Geographical distribution of status of legislations pertaining to interchangeability (A) Europe (B) USA
Regulators approach towards interchangeability issues
Though EMA has already approved various biosimilar products, the issue of interchangeability has been deferred to the regional operators (local regulatory agencies) [24]. In the US, the states have the authority to propose the biosimilar substitution bills in the assembly preceding the availability of the biosimilars in the US market.
The subject of interchangeability still remains tricky owing to its theoretical definitions and on-paper assumptions. However, for the regulatory authorities it is a herculean task to balance the demands from legislators, branded industry, the generics industry, patient groups and safety advocates. Regulators insist that drug companies ensure high levels of safety even when users switch back and forth between a biosimilar and its reference product [23, 29]. Canada has several patient advocacy groups whose efforts have proven to be beneficial in addressing patient issues especially in the context of chronic diseases. Due to serious concerns over safety aspects pertaining to biosimilars, these patient advocacy groups are campaigning for stringent guidelines for interchangeability and substitution [30]. The question on how this issue would be addressed by the companies in adhering to the regulatory norms and guidelines remains wide open.
The most difficult question bothering the regulators is to demonstrate biosimilarity for different kinds of products, such as alpha- and beta-interferon products and other biotechnology-derived proteins. This would require excessive calibration and frame regulations, which historically has been a slow process [31, 32].
Legal issues entwined with interchangeability
The production processes used to manufacture biological product is a major hurdle for any biosimilar manufacturer. The biggest challenge is to determine that products are interchangeable due to their expected resemblance in safety, efficacy and quality. In due course, interchangeability issues are expected to be a major component of litigation as they are prone to be challenged by originators [3].
The originator companies changing their reference products on a continuous basis hinders the biosimilar approval process. This tactic is commonly observed in generic pharmaceuticals and is now employed in biologicals industry as well.
The interlinked patents associated with the product, pose a severe uncertainty for biosimilar manufacturers. The innovator companies have already initiated their campaign to restrict biosimilar manufacture not to use the name of reference product on their label. Although generic manufacturers of small molecules have endured the same turbulence, over the years, the outcome has favoured them in most cases. However, it is still uncertain as to what extent does this principle apply to biosimilars [3].
Varied approaches by different countries to address interchangeability are also roadblocks for biosimilar development. According to Health Canada, the companies must use different names, as it does not recognize interchangeability. Similarly, EMA has its guidance documents for generic-named products [31].
Companies’ focus on technological barriers that affect regulation
To ascertain inter-changeability, several analytical approaches need to be employed. Developing high accuracy analytical tools is of paramount importance to describe the extent of similarity between the innovator and biosimilar product which is currently lacking. A large concern in regulations has been over the heterogeneity that Post Translational Modifications (PTMs) cause between biosimilars and innovator products. Significant progress has been achieved especially in glycol-engineering by companies, such as Glycofi (now acquired by Merck & Co) which has engineered yeast strains capable of producing human like glycosylations [33]. Novel clinical trial designs are also crucial in establishing interchangeability. The recently proposed modified Balaam’s design [34] would facilitate this process. The coming decades would definitely see more technological advances that could be translated to the product level which is a mandatory step in addressing safety concerns frequently expressed by regulators.
Commercial implication
A decentralized approach followed by EMA and FDA in approving interchangeability will narrow the prospects for biosimilars. Lack of specific requirements by individual states or countries for biosimilars interchangeability approval is further adding to the already confused state.
Currently, biosimilars are priced at 40‒60% discount to originator and would also expand the treatable populations. In case of monoclonal antibodies (mAb), their current usage is restricted only to naïve patient segments and this would still not address the cost burden on the state in providing healthcare facilities. The interchangeability will not only bring down the overall healthcare spending particularly in chronic diseases and will also expand the treatable population. However, if no consensus on interchangeability is reached, the current scenario would impact biosimilar developers on commercial note and might also decelerate entire biosimilar development process.
Regulatory bodies should accept that 100% reproducibility with biosimilars is not feasible [4] and establish permissible limits for deviation in structural aspects. With this directional support, regulators could direct the companies to conduct clinical trials in a small population to establish the safety and efficacy of the biosimilars along with the limits for deviations.
Pharmaceutical companies also need to focus on establishing the biosimilar safety and efficacy by carrying out pharmacovigilance/real world evidence studies in the states or local countries where biosimilars have already been approved. This information would be critical to gain regulators’ trust to proceed towards interchangeability.
Conclusion
Establishing interchangeability is an important step to ensure complete acceptance of biosimilars and high market penetration. Currently, several safety issues, such as immunogenecity, intolerance, clearance, prevents regulators from designating a biosimilar as interchangeable. This may be mainly due to lack of clarity in guidelines and regulations owing to technical barriers and complexity associated with the manufacturing process. However, some countries have certain legislations that accept interchangeability at the pharmacy level with a few clauses. The experiment in France would probably serve as an example to other countries to go ahead with similar legislations and guidelines. However, currently the notion of interchangeability is accepted only in small proteins or peptides. Monoclonal antibodies are associated with several complexities and achieving this status seems far from sight. The originator companies, with their incremental innovations change the properties of the molecule periodically, which becomes difficult to designate a reference molecule. With the innovator companies focusing on second-generation molecules and bio-betters, the cost advantage of biosimilars is likely to be further challenged. Since these companies have an advantage of precise technological knowhow, issues pertaining to micro-heterogeneity may be addressed better. Currently, several branded companies entering manufacture of biosimilars, which are established to have technical capabilities and strategies to achieve high market penetration. The biosimilar companies need to extensively focus on pharmacovigilance studies gathering strong real world evidence data to prove comparable response in patients treated with a biosimilar with the reference product. Recent studies on Inflectra do indicate some positive signs in this direction [35]. However, to reach such consensus favouring interchangeability, it is a long way ahead for both the biosimilar companies and the regulatory agencies.
Acknowledgements
The authors thank Mr Barani Kumar for his help in editing the figure.
Funding sources
This paper was prepared and sponsored by phamax AG.
Authors
Phani Kishore Thimmaraju, BPharm; R Rakshambikai, PhD; Raheem Farista, MPharm; Karthaveerya Juluru, MPharm
Author for correspondence: Phani Kishore Thimmaraju, phamax AG, #19, KMJ Ascend 1st Cross, 17th C Main 5th Block, Koramangala, Bangalore 560095, India
References
- Sekhon, BS, Saluja V. Biosimilars: an overview. Biosimilars. 2011;1(1-11).
- GaBI Online - Generics and Biosimilars Initiative. Interchangeability (switching and alternating) of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Biosimilars/Research/Interchangeability-switching-and-alternating-of-biosimilars
- Endrenyi L, Chang C, Chow SC, Tothfalusi L. On the interchangeability of biologic drug products. Stat Med. 2013;32(3):34-41.
- Rakshambikai R, Thimmaraju PK. Technical hindrances in establishing biosimilarity – the final lap in the race. Intl J Innov and Appl Studies. 2015;11(3):728-33.
- Shein-Chung Chow, Ju C. Assessing biosimilarity and interchangeability of biosimilar products under the Biologics Price Competition and Innovation. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(1):20-5. doi:10.5639/gabij.2013.0201.004
- US state legislation on biosimilars substitution. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(3):155-6. doi:10.5639/gabij.2013.0203.040
- Biotechnology Industry Organization. BIO Principles on patient safety in the substitution of biologic products [homepage on the Internet]. 2013 Jan 24 [cited 2015 May 13]. Available from: www.bio.org/advocacy/letters/bio-principles-patient-safety-substitution-biologic-products
- Massechusetts House Bill 3734 (HB 3734). 2014. [cited 2015 May 13]. Available from: www.legiscan.com/MA/text/H3734/id/897861/Massachusetts-2013-H3734-Introduced.pdf
- Delaware Senate Bill 118 (SB 118). [cited 2015 May 13]. Available from: http://legis.delaware.gov/LIS/LIS147.nsf/vwLegislation/SB+118?Opendocument
- Indiana Senate Bill 262 (SB 262). 2014. [cited 2015 May 13]. Available from: www.iga.in.gov/legislative/2014/bills/senate/262/
- Utah Senate Bill 078 (SB 078). 2013. [cited 2015 May 13]. Available from: www.le.utah.gov/~2013/htmdoc/sbillhtm/SB0078.htm
- Benedict AL. State-level legislation on follow-on biologic substitution. J Law Biosci. 2014. doi: 10.1093/jlb/lsu005
- Traynor K. Virginia passes nation's first biosimilar substitution law. Am J Health Syst Pharm. 2013;70(10):834-6
- GaBI Online - Generics and Biosimilars Initiative. Idaho proposes legislation on biosimilars substitution [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Policies-Legislation/Idaho-proposes-legislation-on-biosimilars-substitution
- Washington Senate Bill 5469 (SB 5469). 2013. [cited 2015 May 13]. Available from: www.legiscan.com/WA/text/SB5469/id/715342/Washington-2013-SB5469-Introduced.pdf
- Georgia Senate Bill 370 (SB 370). 2014. [cited 2015 May 13]. Available from: www.legiscan.com/GA/text/SB370/id/954575/Georgia-2013-SB370-Introduced.pdf
- Michigan House Bill 5598 (SB 5598). 2014. [cited 2015 May 13]. Available from: www.legislature.mi.gov/(S(nur03iu2ytrtnl554nigacag))/mileg.aspx?page=getobject&objectname=2014-HB-5598
- Vermont House Bill 0837 (HB 0837). 2013. [cited 2015 May 13]. Available from: www.legiscan.com/VT/text/H0837/id/946367/Vermont-2013-H0837-Introduced.pdf
- Nevada Senate Bill 126 (SB 126). [cited 2015 May 13]. Available from: www.leg.state.nv.us/Session/77th2013/Bills/SB/SB126.pdf
- Mississippi House Bill 1268 (HB 1268). 2014. [cited 2015 May 13]. Available from: www.billstatus.ls.state.ms.us/documents/2014/pdf/HB/1200-1299/HB1268IN.pdf
- Illinois Senate Bill 193 (SB 193). 2014. [cited 2015 May 13]. Available from: http://www.ilga.gov/legislation/BillStatus.asp?DocNum=1934&GAID=12&DocTypeID=SB&LegID=&SessionID=85&SpecSess=&Session=]&GA=98
- GaBI Online - Generics and Biosimilars Initiative. California and Illinois consider biosimilar substitution bills [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Policies-Legislation/California-and-Illinois-consider-biosimilar-substitution-bills> (2015 ).
- McCamish MW. Worldwide experience with biosimilar development. MAbs. 2011;3(2):209-17.
- GaBI Online - Generics and Biosimilars Initiative. Efficacy, extrapolation and interchangeability of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Biosimilars/Research/Efficacy-extrapolation-and-interchangeability-of-biosimilars
- France's biosimilar law may set trend inside the EU. Law360. 2014. [cited 2015 May 13]. Available from: www.law360.com/articles/507058/france-s-biosimilar-law-may-set-trend-inside-the-eu
- GaBI Online - Generics and Biosimilars Initiative. France to allow biosimilars substitution [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Policies-Legislation/France-to-allow-biosimilars-substitution
- European Biopharmaceutical Enterprises. French Biosimilar Law - No generics-style substitution policy. 2014 [cited 2015 May 13]. Available from: www.ebe-biopharma.eu/newsroom/download/54/document/ebe-bs-statement-final_24.01.2014.pdf
- GaBI Online - Generics and Biosimilars Initiative. Use of biosimilars in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Reports/Use-of-biosimilars-in-Europe
- Sensabaugh SM. Requirements for biosimilars and interchangeable biological drugs in the United States—in plain language. Drug Information Journal. 2011;45:155-62.
- Martinusen DJ, Lo C, Marin JG, Tsao NW, Leung M. Potential impact of subsequent entry biologics in nephrology practice in Canada. Can J Kidney Health Dis. 2014; 1:32.
- Gottlieb S. Biosimilars: policy, clinical, and regulatory considerations. Am J Health Syst Pharm. 2008;65(14 Suppl 6):S2-8.
- Zelenetz AD, et al. NCCN Biosimilars White Paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Canc Netw. 2011;9 Suppl 4:S1-22.
- Potgieter TI, et al. Production of monoclonal antibodies by glycoengineered Pichia pastoris. J Biotechnol. 2009;139(4):318-25.
- Lu Y, Chow S, Zhang Z. Statistical designs for assessing interchangeability of biosimilar products. Drug Des. 3:109. doi: 10.4172/2169-0138.1000109
- GaBI Online - Generics and Biosimilars Initiative. Patient registry data supports efficacy and safety of Inflectra [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 May 13]. Available from: www.gabionline.net/Biosimilars/Research/Patient-registry-data-supports-efficacy-and-safety-of-Inflectra
 2
2







Post your comment