The European Union (EU) was the first to establish a legal framework for biosimilars back in 2003 [1]. Since the European Medicines Agency (EMA) approved its first biosimilars in 2006, the agency has issued new guidelines and updated its existing guidelines based on new evidence and rapid advances in analytical sciences [2].
Biosimilars applications reviewed in the EU
Biosimilars/Research
|
Posted 04/10/2019
 0
Post your comment
0
Post your comment

In their article [3], authors from the Paul-Ehrlich-Institut, the European Medicines Agency (EMA) and the Federal Institute for Drugs and Medical Devices (BfArM), discussed the biosimilars applications reviewed by EMA to date.
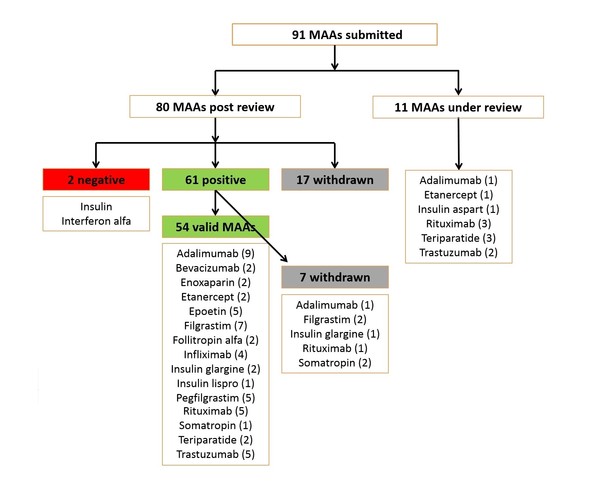
Since EMA started reviewing applications for biosimilars, and up to July 2019, the agency had received 91 marketing authorization applications (MAAs). Of these, 11 were still under review [4] and 17 had been withdrawn. Up to July 2019, the agency had given a positive opinion for 61 MAAs [of which seven were later withdrawn (one for adalimumab, two for filgrastim, one for insulin glargine, one for rituximab and two for somatropin)] and a negative opinion for two MAAs (interferon alfa and insulin), see Figure 1.
Figure 1: Biosimilars reviewed by EMA (to July 2019)
MAAs: marketing authorization applications.
The authors note that ‘the number of biosimilar MAAs that have either been rejected or withdrawn by the company during the CHMP review process could be an indication of the stringent entry barrier for applicants to obtain approval and reach the market’.
Disclaimer
The authors of the research paper [1] declared that the views and opinions expressed in the paper are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agencies with which the authors are affiliated.
Conflict of interest
The authors of the research paper [1] declared that there was no conflict of interest.
Editor’s comment
If you are interested in contributing a research article in a similar area to the GaBI Journal, please send us your submission here.
Related articles
Clinical data requirements for biosimilars in the EU: immunogenicity comparability
Clinical data requirements for biosimilars in the EU: efficacy comparability
Clinical data requirements for biosimilars in the EU: PK and PD comparability
Clinical data requirements for biosimilars in the EU: analytical comparability
Clinical data requirements for biosimilars in the EU
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Oct 4]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
2. GaBI Online - Generics and Biosimilars Initiative. EU guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Oct 4]. Available from: www.gabionline.net/Guidelines/EU-guidelines-for-biosimilars
3. Wolff-Holz E, Tiitso K, Vleminckx C, Weise M. Evolution of the EU Biosimilar Framework: Past and Future. BioDrugs. 2019 Sep 20. doi: 10.1007/s40259-019-00377-y. [Epub ahead of print]
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilars applications under review by EMA – July 2019 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Oct 4]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-applications-under-review-by-EMA-July-2019
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment