Biosimilars
Canada approves ranibizumab biosimilar Byooviz
The ranibizumab biosimilar Byooviz (SB11), produced by Samsung Bioepis and commercialized by Biogen, received approval from Canada’s drug regulator, Health Canada, on 8 March 2022. This is the first ranibizumab biosimilar to receive approval in Canada.
FDA accepts application for interchangeability of adalimumab biosimilar Abrilada
US-based pharma giant Pfizer announced on 25 February 2022 that the US Food and Drug Administration (FDA) had accepted for review the Prior Approval Supplement (PAS) to the Biologics License Application (BLA) for Abrilada (adalimumab-afzb) as an interchangeable biosimilar to Humira (adalimumab).
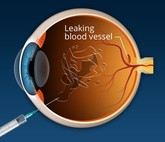
The CROWN study: real-world comparison between ‘similar biologic’ and originator ranibizumab and bevacizumab
The last decade and a half have witnessed a paradigm shift in the way vascular disorders affecting the retina are treated. Anti-vascular endothelial growth factor (VEGF) antibodies have become the mainstay of treatment. These monoclonal antibodies are injected in the vitreous cavity. For the treatment to be effective, repeated injections every 4 to 8 weeks over many years need to be given, which proves to be quite expensive.
Will the unclear path of biosimilar interchangeability become clearer?
Biosimilar use has led to increased access to biological treatments for some molecules wherein switching from originator to biosimilar has been one means to exchange products [1, 2]. Another but less applied way to exchange the products is via substitution by a pharmacist, i.e. without informing the prescriber [2]. Substitution of biosimilars is a topic with many contrasting views and has been highly debated [3-12]. More clarity on the matter is needed because health care has reached a point where policy decisions need to be made about whether substitution should become a practice in biosimilar use. Therefore, researchers from Denmark, Sweden and The Netherlands investigated the views of experts from medicines agencies and the pharmaceutical industry on the science underpinning interchangeability of biosimilars [13].
Biosimilars approved in Argentina
In Argentina, the regulatory agency in charge of approving biological drugs is the National Administration of Drugs, Food and Medical Devices (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica, ANMAT).
Generium launches omalizumab (Xolair) non-originator in Russia
Moscow’s Generium has launched a non-originator biological of omalizumab (Xolair) on the Russian market, the third of Generium’s products using Selexis’ SUREtechnology Platform to reach the market.
Europe’s IP framework should support earlier authorization of biosimilars, review finds
A review of the European Pharmaceutical Strategy suggests the framework should focus on intellectual property (IP) initiatives that support the earlier authorization of biosimilars.
Key considerations when switching to biosimilar insulin
Biosimilar insulins can offer a cheaper alternative to originator insulators and substantial savings for healthcare systems. A recent ‘Quick guide: Initiating or switching to a biosimilar insulin’, [1] published in the Journal of Diabetes Nursing, outlines key aspects to consider when switching.
Biosimilars and non-medical switching among Saudi rheumatologists. The knowledge gap
The increasing cost of originator biologicals, combined with consequences of the COVID-19 pandemic, have burdened the healthcare system. Unfortunately, the biosimilar uptake in Saudi Arabia has not reached an optimal level despite the approval of several agents. Therefore, physicians are essential stakeholders in the process and acceptance of biosimilars.
Nova Scotia, Canada implements biosimilar switching policy
Nova Scotia becomes the fifth Canadian province to implement a biosimilar switching policy, meaning that half of Canada’s provinces have now implemented such policies.