Biosimilars
Potential biotech collaboration between India and Colombia
A letter of intent on cooperation in biotechnology has been signed between India and Colombia, which could include collaborations on vaccines, biosimilars, and medical devices.
Biosimilars approved in Paraguay
In Paraguay, the regulatory body responsible for the approval of biological drugs is the National Directorate for Sanitary Surveillance (Dirección Nacional de Vigilancia Sanitaria, DINAVISA).
Positive trial results for bevacizumab copies from Betta Pharmaceuticals and Biocad
The bevacizumab copy biological MIL60 (Betta Pharmaceuticals Ltd) and non-originator biological BCD-021 (Biocad) have demonstrated clinical equivalence for the treatment of non-squamous non-small cell lung cancer (NSCLC) in phase III trials.
CuraTeQ submits application to EMA for pegfilgrastim biosimilar
India-based generics maker Aurobindo Pharma (Aurobindo) announced on 22 September 2021 that its subsidiary CuraTeQ Biologics (CuraTeQ) had submitted an application to the European Medicines Agency (EMA) for its pegfilgrastim biosimilar, BP14.
Candidate trastuzumab biosimilar AryoTrust
Biologicals are one of the interesting and effective treatment options which can save the lives of many patients, however, their high cost and restricted access for some patients remains a challenge. The emergence of biosimilars, with their similar efficacy and safety profiles, could be a solution for this hurdle. According to the European Medicines Agency (EMA) guidance entitled ‘Biosimilars in the EU – information guide for healthcare professionals’, a biosimilar is ‘a biological medicine highly similar to another biological medicine already approved in the EU’ [1]. Biosimilars are required to have the same standards of pharmaceutical quality, safety and efficacy as for originator biologicals in order to obtain marketing authorization. Although, according to the EMA guidance ‘approval of biosimilars builds on existing scientific knowledge on safety and efficacy of the reference medicine gained during its clinical use, so fewer clinical data are needed’ [2].
Biosimilars approved in Cuba
In Cuba, the regulatory body responsible for approving biological drugs is the Center for the State Control of Medicines, Medical Equipments and Devices (Control Estatal de Medicamentos, Equipos y Dispositivos Médicos, CECMED).
China approving more copy biologicals since new guidelines introduced
China has approved many more copy biologicals in the last three years. A move which, according to Pharmaceutical Technology, is thought could be due to the introduction of new guidelines for the products.
Barriers to biosimilar prescribing incentives in Spain
Incentives contribute to the proper functioning of the broader contracts that regulate the relationships between healthcare systems and professionals. Likewise, incentives are an important element of clinical governance understood as healthcare services’ management at the micro-level, aimed at achieving better health outcomes for patients.
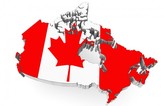
Canada’s biosimilar substitution policy: effects on competition and patient safety
A critical review of Canada’s biosimilar substitution policy [1] finds that the scheme has focused on economic factors over other elements such as therapeutic efficacy and market competition. The authors suggest that Canada could learn from the European market, where switching policies retain choice for physicians and patients and promote competition.
Biosimilars approved in Peru
In Peru, the regulatory body responsible for approving biological drugs is the General Directorate of Medicines, Supplies and Drugs (DIGEMID) of the Peruvian Ministry of Health (MINSA) which is in charge of leading the National and Decentralised Health System, the policy for the universal health assurance and the policies and intersectoral actions on social determinants [1].