Biosimilars
Biosimilars applications under review by EMA – January 2020
The European Medicines Agency (EMA) is the body responsible for approval of biosimilars within the European Union (EU). A legal framework for approving biosimilars was established in 2003. Approval of biosimilars is based on an abbreviated registration process, which allows biosimilars manufacturers to provide a reduced package of information compared to originator drugs, provided they can prove ‘similarity’ to the originator or reference drug.
Mabpharm files infliximab copy biological application in China
US-based biopharmaceutical company Sorrento Therapeutics (Sorrento) announced on 6 January 2020 that its partner, China-based Mabpharm, had filed a marketing application with China’s National Medical Products Administration (NMPA), formerly the China Food and Drug Administration (CFDA) for its infliximab copy biological (CMAB008).
Adalimumab biosimilars in Europe: a review
A recent position statement from the Belgian inflammatory bowel disease (IBD) research group (BIRD) reviews the five biosimilars of adalimumab available in the European Union (EU) [1]. Adalimumab, which has been marketed under the brand name Humira, is a popular antibody treatment for a number of inflammatory conditions.
China approves bevacizumab copy biological Ankeda
China’s National Medical Products Administration (NMPA), formerly the China Food and Drug Administration (CFDA), announced on 9 December 2019 that it had approved Ankeda, a bevacizumab copy biological.
Swiss position statement on the use of biosimilars in IBD
Experts from the Swiss Society of Gastroenterology have provided a position statement on the use of biosimilars in inflammatory bowel disease (IBD). The position paper summarizes key regulatory and clinical issues around biosimilars to help improve clinician and patient awareness.
Teriparatide biosimilar Terossa approved in South Korea
South Korea-based Daewon Pharmaceutical has obtained regulatory approval to begin marketing and selling its teriparatide biosimilar in Korea.
Eli Lilly and Novo Nordisk launches lower-priced insulin options
Originator insulin makers Eli Lilly and Novo Nordisk have both announced the introduction of additional reduced cost insulin options for patients.
Bevacizumab and teriparatide biosimilars launched in Japan
Biosimilars for osteoporosis treatment teriparatide and for anticancer drug bevacizumab were launched in Japan in late 2019.
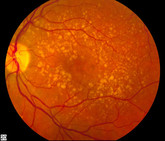
Real-life clinical effectiveness of Razumab in wet AMD
The multicentre, retrospective RE-ENACT 2 study evaluated the real-world effectiveness of ranibizumab biosimilar, Razumab, for the treatment of wet age-related macular degeneration (wet AMD). This report presents the results from a subgroup analysis of the RE-ENACT 2 study [1].
WHO prequalifies first biosimilar
The World Health Organization (WHO) has prequalified its first biosimilar – trastuzumab – in a move that the organization says, ‘could make this expensive, life-saving treatment more affordable and available to women globally’.