Maryland has become the fourth US state to reject legislation that could restrict pharmacists’ ability to substitute cheaper biosimilars for their reference biologicals.
Fourth US state rejects law restricting biosimilar substitution
Home/Policies & Legislation
|
Posted 12/04/2013
 1
Post your comment
1
Post your comment
On 8 April 2013, Members of the House in Maryland rejected a law requiring ‘unnecessary notification and record keeping’ by pharmacists when substituting biosimilars. Actions that the Generic Pharmaceutical Association (GPhA) says ‘would create red tape between patients and affordable medicines’.
Three other states: Arizona, Mississippi and Washington have also rejected similar legislation. While Arkansas concluded that this issue warranted further research, and sent their legislation to a study committee.
Nine other states: California, Colorado, Illinois, Indiana, Florida, Massachusetts, Oregon, Pennsylvania and Texas, however, are still considering proposals to restrict the substitution of biosimilar drugs for brand-name biologicals. North Dakota has passed legislation requiring notification and record keeping for biosimilars. Virginia and Utah have also passed such a law, but with a sunset clause, which means that the bill is likely to expire before any biosimilars are approved in the US.
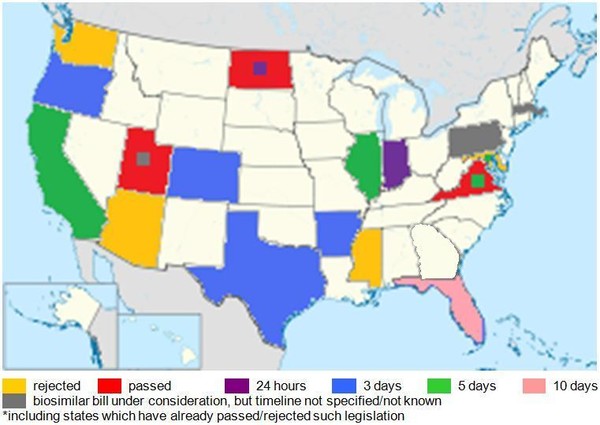
Most of the proposed bills require the pharmacist to notify the prescribing physician of a biosimilar substitution, but the timelines for notification vary from within 24 hours in Indiana to within 10 days in Florida, see Figure 1.
Figure 1: US states with legislation under consideration for biosimilar substitution and timelines for physician notification*
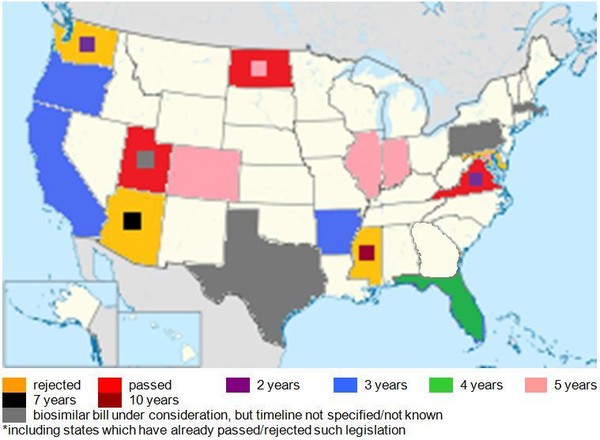
Most of the legislation under consideration also requires the pharmacist to retain a record of the biosimilar substitution, but again the length of time that such records have to be kept varies from two to 10 years, see Figure 2.
Figure 2: US states with legislation under consideration for biosimilar substitution and record-keeping periods*
Originator biotechnology companies have argued that such legislation will protect patient safety and should be used as a model for all 50 US states. The GPhA on the other hand is sticking to its view that any laws concerning biosimilar substitution are ‘premature and unnecessary at this time’.
Related article
US state biosimilar substitution bill becomes law
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
Source: GPhA
Posted 15/05/2013 by Christina
Washington Bill
It looks like the Washington house of reps reintroduced HB 1528 yesterday for its special session. Not so dead after all?
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
EU accepts results from FDA GMP inspections for sites outside the US
Home/Policies & Legislation Posted 27/01/2026
WHO to remove animal tests and establish 17 reference standards for biologicals

Home/Policies & Legislation Posted 07/01/2026
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment