Although overall a large number of producers supply the drugs on the shortages list in the US, most of the drugs are supplied by only one or two companies according to a new report published by IMS Institute for Healthcare Informatics (IMS Institute) [1].
Suppliers of products on the drug shortages list in the US
Home/Reports
|
Posted 03/02/2012
 0
Post your comment
0
Post your comment

In total there are over 100 suppliers involved in supplying drugs on the shortages list, however, 50 of these drugs have only one supplier, while two-thirds of the drugs have three or fewer suppliers.
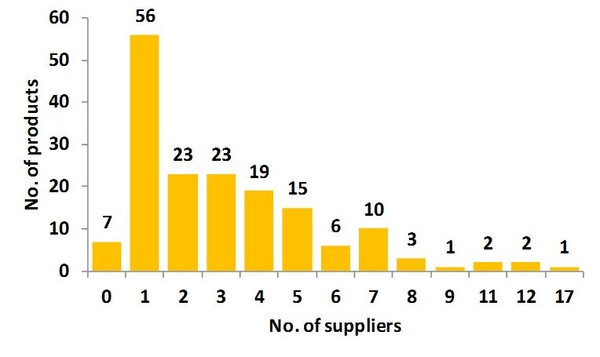
Of the 168 products on the current shortages list, seven currently have no suppliers, while 56 products have one supplier, 23 have two suppliers and another 23 have three suppliers, see Figure 1.
Figure 1: Number of products by supplier on the drug shortages list in the US
Source: IMS Institute for Healthcare Informatics [1]
Generic drugs not on the shortages list are far more likely to have multiple suppliers. In the overall generic pharmaceuticals market, 31% of the 1,026 molecules have two or fewer suppliers compared to 36% of generic injectables which have two or fewer suppliers. This increases to 51% for the 168 drugs on the current shortages list.
Sterile injectables have specific problems since manufacturing such products is complex and can more easily lead to problems that affect safety. They also often require dedicated manufacturing lines. The top three generic injectable manufacturers hold 71% of the market by volume. Most sterile injectables have just one manufacturer that produces at least 90% of the drug–innovator and generic combined [2].
The number of companies supplying the drugs on the shortages list has fluctuated over the last five years with an increasing number of companies no longer supplying the products on the shortages list. This is mainly due to economic reasons. If drug makers do not make enough profit they will not make generic drugs [2].
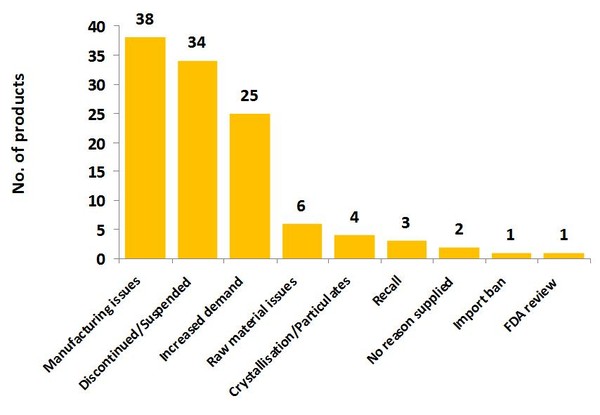
The main reasons given by suppliers for drug shortages on the list were manufacturing issues (38 products), the product being discontinued or suspended (34 products) and increased demand (25 products), not raw material or quality issues.
Issues related to specific quality concerns, such as crystallisation or particulates in injectables, or which have resulted in product recalls, were cited seven times, see Figure 2.
Figure 2: Reason for shortages of drugs in the US
Source: IMS Institute for Healthcare Informatics [1]
Related articles
Early warning system for drug shortages in the US
Volume and sales of drugs on the shortages list in the US
Which drugs are affected by drug shortages in the US
Investigating drug shortages in the US
References
1. IMS Institute for Healthcare Informatics. Drug Shortages: A closer look at products, suppliers and volume volatility. November 2011.
2. GaBI Online - Generics and Biosimilars Initiative. Cancer drug shortages in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 Feb 03]. Available from: http://www.gabionline.net/Pharma-News/Cancer-drug-shortages-in-the-US
Source: IMS
Policies & Legislation
ANVISA and Danish Medicines Agency renew health regulatory collaboration
Colombia and Brazil introduce reforms to enhance healthcare regulation
China-to-West pharma licensing deals surge in 2024 amid innovation push

Home/Reports Posted 22/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment