Despite record numbers of drug shortages being reported in the US [1], on the whole, over the past five years the supply of these drugs to healthcare providers–hospitals, clinics, pharmacies–has increased, according to a new report published by IMS Institute for Healthcare Informatics [2].
Volume and sales of drugs on the shortages list in the US
Home/Reports
|
Posted 10/02/2012
 0
Post your comment
0
Post your comment

In fact, for over half of the drugs on the shortages list, total supply is relatively stable or has increased over the past five years. However, there has been a dramatic decline in the total supply available for the remaining drugs on the shortages list, see Figure 1.
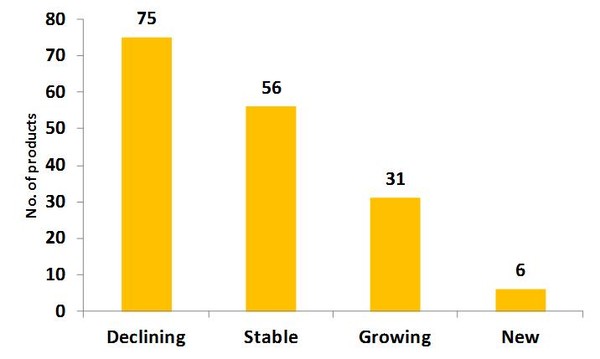
Figure 1: Variation of supply for drugs on the shortages list in the US
Source: IMS Institute for Healthcare Informatics [2].
Among the 168 drugs on the drug shortages list maintained by FDA and American Society of Health-System Pharmacists (ASHP) as of 7 October 2011, distinct supply patterns have emerged during the three months ending August 2011 compared to the average monthly volume in 2006–09:
- Declining: 75 drugs declined by more than 20% in supply
- Stable: 56 drugs are within 80–120% of historical supply levels
- Growing: 31 drugs have seen volume increases of over 20%
- New: 6 drugs were newly placed on the list and no trend was yet evident
Spending for drugs on the shortages list has also increased. However, the market is now showing high volatility, with unusually sharp swings in supply for many of the drugs on the shortages list.
Within the different US states utilisation also varies significantly, with 13 states experiencing significant drops in supply.
Many believe that these swings and differences in supply and price may be due to the practice of hoarding of essential drugs on the shortages list and the subsequent grey market, which also results in inflated prices for drugs in short supply [3].
Related articles
Early warning system for drug shortages in the US
Suppliers of products on the drug shortages list in the US
Which drugs are affected by drug shortages in the US
Investigating drug shortages in the US
References
1. GaBI Online - Generics and Biosimilars Initiative. Some relief from drug shortages in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Pharma-News/Some-relief-from-drug-shortages-in-the-US
2. IMS Institute for Healthcare Informatics. Drug shortages: a closer look at products, suppliers and volume volatility. 2011 Nov.
3. Kantarjian HM. Chemotherapy drug shortages: a preventable human disaster. ASCO Blog Post. 2011 Nov 15;2:(17).
Policies & Legislation
ANVISA and Danish Medicines Agency renew health regulatory collaboration
Colombia and Brazil introduce reforms to enhance healthcare regulation
China-to-West pharma licensing deals surge in 2024 amid innovation push

Home/Reports Posted 22/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment