Use of biosimilars in Madrid, Spain, has increased after approaches were introduced to try and improve uptake of biosimilars in the country, according to Ainhoa Aranguren Oyarzábal and colleagues from the Madrid Health Service (MHS), Spain [1].
In 2010, the Assistance Direction of Pharmaceutical Management (ADPM) established a strategic approach to improve the uptake of biosimilars in Madrid. Biosimilars use was monitored via the use of indicators, which were included in the Contract Plan – an agreement signed between the MHS and hospitals in the region. In 2014, the indicator %Biosimilar Drugs Cost Value was included, and in 2016 the percentage of patients on infliximab biosimilar (new patients) was included.
%Biosimilar Drugs Cost Value = Purchase cost of biosimilars of somatropin, erythropoietin or filgrastim/Purchase cost of all brands of somatropin, all brands or erythropoiesis stimulating agents or all brands of colony-stimulating factors
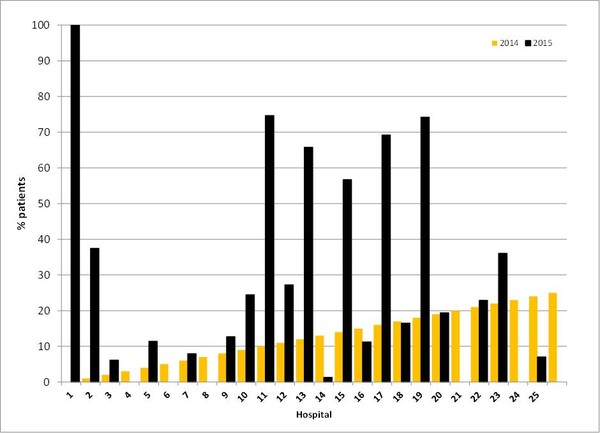
The %Biosimilar Drugs Cost Value for the main hospitals in Madrid was 15.72% in 2014 and 20.07% in 2015. These percentages varied between individual hospitals and groups of hospitals, although most hospitals reported an increase between 2014 and 2016 in the use of biosimilars. The medium size II hospitals (#16–25) reported the greatest uptake of biosimilars (2014: 34.15% vs 2016: 35.46%), followed by medium size I hospitals (#9–15, 2014: 27.59% vs 2016: 29.51%) and large hospitals (#1–8: 2014: 8.69% vs 2016: 13.15%), see Figure 1. A table showing the individual results per hospital can be viewed in the GaBI Journal article [1].
Figure 1: %Biosimilar Drugs Cost Value for hospitals in Madrid in 2014 and 2015
When this indicator was analysed for each drug group (somatropin, colony-stimulating factors and erythropoiesis stimulating agents), the largest uptake of biosimilars was found to be for colony-stimulating factors (50.34%), followed by erythropoiesis stimulating agents (15.04%) then somatropin (7.19%).
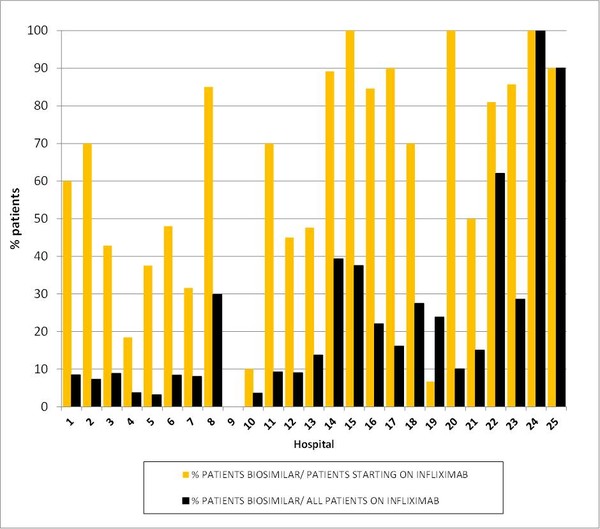
With respect to infliximab indicators, more than half of the patients that started an infliximab therapy in 2015 did so with a biosimilar (290 out of 536 or 54.10%). Considering all patients who received an infliximab prescription, 12.83% (343 out of 2,673 patients) were treated with a biosimilar. These percentages varied between individual hospitals and groups of hospitals. The medium size II hospitals reported the greatest uptake of biosimilar infliximab (% starting: 69.90% vs % infliximab: 35.23%), followed by medium size hospitals (% starting: 54.31% vs % infliximab: 12.70%) and big hospitals (% starting: 48.90% vs % infliximab: 8.53%), see Figure 2. A table showing the individual results per hospital can be viewed in the GaBI Journal article [1].
Figure 2: Percentage of biosimilar patients on infliximab
Oyarzábal and colleagues concluded that as a result of the ADPM initiative the %Biosimilar Drugs Cost Value improved from 15.72% in 2014 to 20.07% in 2015. However, they add that this value could be higher if only biosimilars were taken into account (somatropin, filgrastim, epoetin) instead of the whole group (somatropin, colony-stimulating factors and erythropoiesis stimulating agents), according to the indicator definition’.
Acknowledgement
This article is prepared based on the paper entitled ‘Best practice to improve biosimilars uptake: the experience of Madrid, Spain’ by Aranguren Oyarzábal A, López Centeno B, Alonso Castro V, Calvo Alcántara MJ, Cruz Martos E, Assistance Direction of Pharmaceutical Management, Madrid Health Service, Spain
Editor’s comment
Readers interested to learn more about market uptake of biosimilars are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
Product naming, pricing, and market uptake of biosimilars
Readers interested in contributing a research or perspective paper to GaBI Journal – an independent, peer reviewed academic journal – please send us your submission here.
Related articles
Improvement in uptake of biosimilars in Spain
Approaches to increase uptake of biosimilars in Spain
A strategic approach to increase uptake of biosimilars in Spain
Improving uptake of biosimilars in Spain
Reference
1. Derbyshire M. Improving biosimilars uptake: experience gained in Madrid, Spain. Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(2):89-91. doi:10.5639/gabij.2016.0502.021
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2016 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment