There is obviously a market for biosimilars. This is driven by the cost savings to be made by payers and patients alike. By 2015, IMS Health (IMS) expects spending on biosimilars to exceed US$2 billion annually, or about 1% of total global spending on biologicals. They expect new biosimilars to enter the US market by 2014 and European markets to have additional biosimilar molecules introduced during this period [1].
The biosimilars landscape
Biosimilars/Research
|
Posted 23/09/2011
 0
Post your comment
0
Post your comment
Biosimilars have been on the market since 2006 and are already present in many countries worldwide, see Table 1 [2].
Table 1: Biosimiars market entry onto multiple countries
| Country | HGH | EPO | G-CSF |
| Europe (big 5) | |||
| France | Q2 2007 | Q1 2009 | Q1 2009 |
| Germany | Q1 2007 | Q1 2009 | Q4 2008 |
| Italy | Q2 2006 | Q4 2007 | Q2 2009 |
| Spain | Q3 2007 | Q1 2009 | Q1 2009 |
| UK | Q2 2007 | Q1 2009 | Q4 2008 |
| Europe (rest) | |||
| Austria | Q2 2009 | Q3 2008 | Q1 2009 |
| Belgium | Q2 2009 | ||
| Bulgaria | Q4 2009 | ||
| Denmark | Q1 2010 | Q1 2009 | |
| Finland | Q1 2008 | Q3 2008 | Q3 2009 |
| Greece | Q1 2009 | Q2 2009 | |
| Hungary | Q1 2009 | Q1 2009 | |
| Ireland | Q3 2008 | Q4 2009 | |
| Latvia | Q2 2009 | Q1 2009 | |
| Lithuania | Q1 2010 | Q1 2009 | |
| Netherlands | Q3 2009 | Q4 2009 | Q2 2009 |
| Norway | Q4 2008 | Q1 2009 | |
| Poland | Q2 2009 | Q3 2009 | Q3 2009 |
| Romania | Q3 2008 | Q3 2009 | Q2 2009 |
| Slovakia | Q2 2009 | ||
| Slovenia | Q4 2009 | Q1 2009 | Q4 2009 |
| Sweden | Q2 2008 | Q2 2008 | Q2 2009 |
| Rest of the world | |||
| Australia | Q1 2010 | Q1 2010 | |
| Canada | Q3 2009 | ||
| Japan | Q3 2009 | ||
| US | Q1 2007 |
EPO: erythropoietin; G-CSF: granulocyte colony-stimulating factor; HGH: human growth hormone
Source: IMS Health
The first biosimilars approved were human growth hormone, followed by erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF).
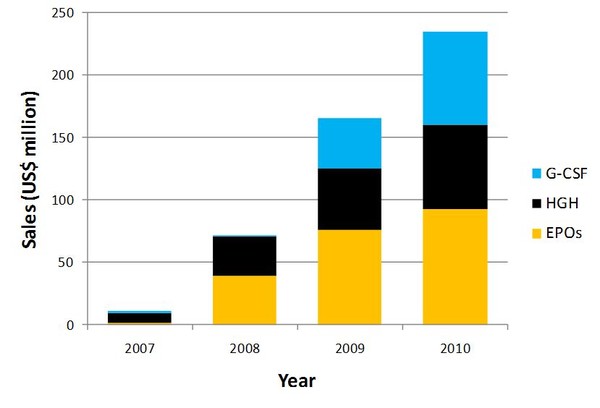
Global sales of biosimilars have more than doubled each year since 2007, and had reached more than US$234 million in 2010, see Figure 1. EPOs take the major share of the market with sales of US$93 million, followed by G-CSF (US$75 million) and human growth hormone (US$67 million). Despite the dominance of EPOs, Sandoz’s Omnitrope—a human growth hormone—is still the largest selling biosimilar, with global sales in 2010 of US$66 million.
Figure 1: Global biosimilar sales of EPO, G-CSF and HGH
EPOs: erythropoietins; G-CSF: granulocyte colony-stimulating factor; HGH: human growth hormone
Source: IMS Health
The biosimilars market is clearly experiencing rapid growth and looks set to reach IMS’s predictions exceeding US$2 billion annually by 2015.
Related articles
The market for global and European biosimilars
Biosimilars and the pharmaceutical industry
Biobetters rather than biosimilars
References
1. GaBI Online - Generics and Biosimilars Initiative. Generics and biosimilars to drive down drug spending [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 September 23]. Available from: www.gabionline.net/Pharma-News/Generics-and-biosimilars-to-drive-down-drug-spending
2. Sheppard A. Biological/Biotechnological and Biosimilars market: the global outlook. 9th EGA International Symposium on Biosimilar Medicines; 2011 Apr 14; London, UK.
News
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment