Generics/News
|
Posted 11/01/2019
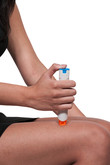
Sandoz, the generics division of Novartis, will launch Adamis Pharmaceuticals Corp’s EpiPen (epinephrine) generic Symjepi in early 2019. Symjepi received US Food and Drug Administration (FDA) approval of its 0.3 mg pre-filled single dose syringe in June 2017 [1], and for its lower dose version (0.15 mg) in September 2018. Epinephrine injectors are used for the emergency treatment of life-threatening allergic reactions (anaphylaxis) caused by allergens, exercise or unknown triggers. These can include reactions to insect bites or stings, foods, medications, latex or other causes.