Biosimilars are now a reality. The European Medicines Agency (EMA) approved its first biosimilar back in 2006 [1] and, with the increasing price of new biologicals and continuing pressure on healthcare budgets, biosimilars are expected to make up an increasing share of the biologicals market.
There are also more than 200 new biotechnology products in the pipeline (phase II to registered), all of which could be future targets for biosimilars.
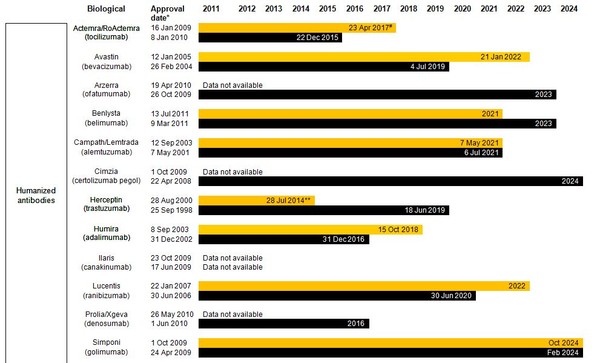
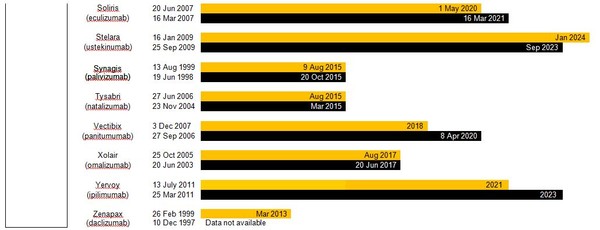
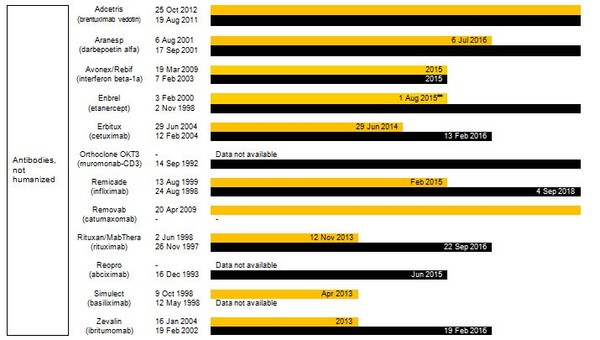
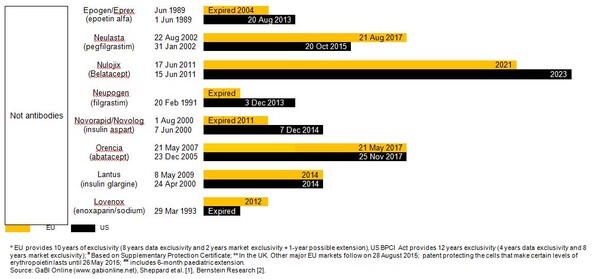
Estimated patent expiry dates for just some of the best-selling biological molecules are shown in Figure 1.
Figure 1: Patent expiry dates on best-selling biologicals
Although the European Union (EU) previously defined a period of 10 years data exclusivity, this was revised at the latest review of pharmaceutical EU legislation to the following:
Ten years if the reference product is centrally approved or application to the centralized procedure has been made before 20 November 2005. Or eight years data exclusivity + 2 years market exclusivity + 1 year possible extension if a full dossier is submitted on or after 30 October 2005 via a national procedure or after 20 November 2005 via the centralized procedure [3].
The expiration of patents and other intellectual property rights for originator biologicals over the next decade opens up opportunities for biosimilars to enter the market and increase industry competition. Price reduction strategies should increase adoption among physicians and patients alike, spurring increases in the biosimilars market share.
According to a report by Frost & Sullivan the global market for biosimilars will rapidly expand more than 20-fold in the next five years. The biosimilars market is expected to increase from just US$1.2 billion during 2013 to US$23 billion in 2019. Demand is being fuelled by governments around the world turning to biosimilars as a cheaper option to reduce healthcare costs. Government initiatives, strategic collaborations and increasing incidents of new diseases are also increasing the value of the market. Deeper penetration into the markets in Europe, Japan and the US, as well as emerging economies in Asia and Latin America is also helping to increase the biosimilars market share.
Major companies investing in the biosimilars market include Amgen, Biocon, Biopartners, Dr Reddy’s Laboratories, Hospira, Intas Biopharmaceultical, Mylan, Sandoz and Teva.
Editor's comment
If you would like to receive a high resolution copy of Figure 1*, please send us an email.
*For profit organizations subjected to a fee
Related articles
Biosimilars: key players and global market trends
2013’s biggest patent expiries
2012’s biggest patent expiries
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Nov 13]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.








 1
1











Post your comment