FDA issued its long-awaited guidance for biosimilar applications on 10 February 2012 in the form of three draft guidance documents [1]. In a webinar on 15 February 2012 FDA discussed some of the issues surrounding biosimilar products and expanded on some of the requirements contained within the guidance documents.
In her presentation, Dr Rachel E Sherman, Associate Director for Medical Policy of the Center for Drug Evaluation and Research, reiterated that according to the Biologics Price Competition and Innovation (BPCI) Act, a biosimilar [351(k)] application must include information demonstrating that the biological product:
- is biosimilar to a reference product;
- utilises the same mechanism(s) of action for the proposed condition(s) of use–only to the extent known for the reference product;
- condition(s) of use proposed in labelling have been previously approved for the reference product; and
- has the same route of administration, dosage form and strength as the reference product.
Biosimilarity
Biosimilarity means:
- that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components; and
- that there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product.
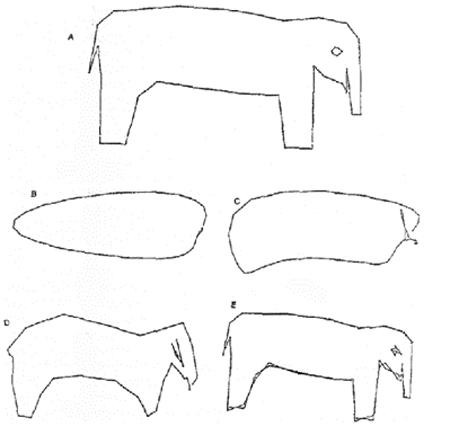
Dr Sherman demonstrated this concept with an illustration of an elephant, see Figure 1, with each line representing a clinical study. She reiterated the fact that FDA would require a ‘totality of evidence’ approach and considers repetition of any animal or human studies to be unethical, therefore, this should be avoided. Using such an approach, FDA would then consider how close the proposed biosimilar products (Figure 1B-E) compared to the reference product, see Figure 1A. Dr Sherman added that ‘advances in current state-of-the-art analytical methods enhance the likelihood that a product will be highly similar to another product by better targeting the original product’s physicochemical and functional properties.’
Some have criticised the guidance documents for being too vague. However, FDA made it clear in this webinar that it expects the minimum characterisation outlined in the guidance documents to be performed up front, and then the biosimilar applicant should contact FDA. At this stage it will then be determined–on a case-by-case basis–what further studies will be required.
Figure 1: Least squares fitting of an elephant
Source: Wei J. Least squares fitting of an elephant. Chemtech. 1975:Feb;128-9.
Studies required for a biosimilar [351(k)] application must include information demonstrating biosimilarity based on data derived from:
- analytical studies demonstrating that the biological product is ‘highly similar’ to the reference product notwithstanding minor differences in clinically inactive components;
- animal studies (including the assessment of toxicity); and
- a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity and potency in one or more appropriate conditions of use for which the reference product is licensed.
Once again it was made clear that there would be no ‘one size fits all’ assessment and that FDA may use its discretion in deciding whether all of the above studies are required for a particular biosimilar.
Interchangeability
The BPCI Act also gives FDA the authority to designate a biosimilar as interchangeable with its reference product. This means that the biosimilar may be substituted for the originator product by the pharmacist without reference to the prescribing physician [2].
This is somewhat different to the situation in the EU, where EMA does not have the authority to designate biosimilars as being interchangeable with the reference product [2].
When FDA published its biosimilars guidance the agency stated that ‘at this time, it would be difficult as a scientific matter for a prospective biosimilar applicant to establish interchangeability in an original 351(k) application’ due to the ‘sequential nature of that assessment’. FDA added that it ‘is continuing to consider the type of information sufficient to enable FDA to determine that a biological product is interchangeable with the reference product’.
The agency also stated that it would use a ‘stepwise approach’ requiring first biosimilarity and then interchangeability to be proved. This was reiterated in Dr Sherman’s presentation. This means that FDA will not provide guidance on how to prove interchangeability until after the first biosimilar is approved. Dr Sherman did however state that FDA will ‘invariably’ require at least one human clinical study to show interchangeability once biosimilarity is established.
Biosimilar proposals
Dr Sherman also gave some information on the number and status of biosimilar proposals that were under discussion at FDA.
FDA has received 35 requests for pre-Investigational New Drug (IND) Application meetings for biosimilars covering 11 different reference products. This is interesting to compare with Europe, where to date 14 biosimilars have been approved covering only three reference products. FDA confirmed that it had already carried out 21 pre-IND sponsor meetings and has received nine INDs.
It seems therefore that the lack of detail contained within FDA’s guidance documents has not put off biosimilars manufacturers and it is only a matter of time before we have the first official biosimilar approved in the US.
Related article
Interchangeability or substitution of biosimilars
References
1. GaBI Online - Generics and Biosimilars Initiative. FDA finally issues draft biosimilar guidance [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Mar 2]. Available from: www.gabionline.net/Guidelines/FDA-finally-issues-draft-biosimilar-guidance
2. GaBI Online - Generics and Biosimilars Initiative. Interchangeability of biosimilars in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Mar 2]. Available from: www.gabionline.net/Biosimilars/News/Interchangeability-of-biosimilars-in-the-US








 0
0











Post your comment