Dr Elena Wolff-Holz* discussed the importance of European public assessment reports (EPARs) at a major biosimilar medicines conference in London, UK [1].
EPARs and Summary of Product Characteristics (SmPCs) are used in the European Union (EU) to provide information on the quality testing for a biosimilar, as well as the non-clinical and clinical information.
EPARs are full scientific assessment reports of medicines authorized at an EU level. They contain information on the pharmacokinetics, safety, immunogenicity and interchangeability studies on biologicals and biosimilars, which are published by the European Medicines Agency (EMA) on their website: www.ema.europa.eu in the form of an EPAR.
SmPC is the basis of information for healthcare professional on how to use the medicine. It provides essential information for the use of a medicine. This includes qualitative and quantitative information on the benefits and the risks, information for individualized care, information on specific situations and pharmaceutical information.
In January 2017, the European Society for Medical Oncology (ESMO) published a position paper on biosimilars [2]. In it they state that ‘in the case of biosimilars for monoclonal antibodies (mAbs), the submitted information from the clinical studies, including detailed pharmacovigilance plans, needs to be reflected on the label’. This they say ‘is important in the field of oncology, as physicians should be suitably informed regarding:
(1) the patient population it was tested on, i.e. a sensitive patient population
(2) the sensitivity of endpoints used in the trial to demonstrate the efficacy of the biosimilar for the specific indication.
Thus, the label or SmPC should clearly reflect the information concerning the product it contains and refer to the appropriate sections of EPAR that are important considerations for the physician. As a biosimilar is not an identical clone of the originator biological, data concerning the extrapolation, interchangeability, switching and automatic substitution, immunogenicity and traceability should also be detailed appropriately.
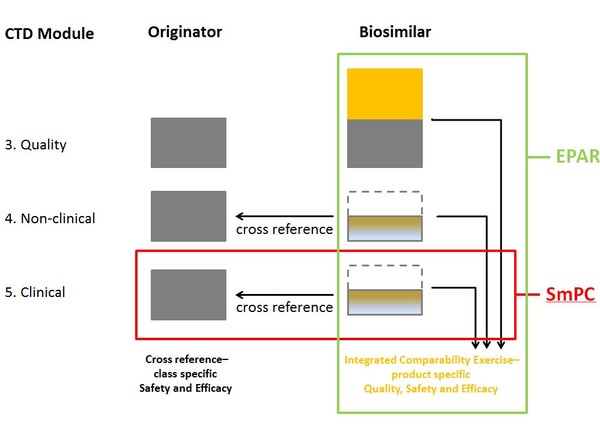
Dr Wolff-Holz highlights the information contained in EPARs compared to that in SmPCs. SmPC includes clinical information on both the originator biological and the biosimilar. EPAR, on the other hand, includes all the quality, non-clinical and clinical data for the biosimilar, as well as information on the integrated comparability exercise and product specific quality, safety and efficacy, see Figure 1.
Figure 1: Information contained in EPAR versus SmPC
CTD: Common Technical Document; EPAR: European public assessment report; SmPC: Summary of Product Characteristics.
There is therefore a need to increase the emphasis/strengthen the:
- Quality aspects (CQAs) – Comparability exercise
- Justification of extrapolation as a result of positive benefit/risk
CQA: critical quality attribute.
Toward this end EMA issued a proposal for the inclusion of additional biosimilarity data (quality) in EPAR in March 2017.
*Dr Wolff-Holz works at the Paul Ehrlich Institut, Federal Agency for Vaccines and Biomedicines, and is Chair of the Biosimilar Medicinal Products Working Party of the European Medicines Agency’s Committee for Medicinal Products for Human Use.
Related articles
Is a biosimilar forever or just for Christmas?
Biosimilars in the European Union
References
1. Wolff-Holz E. Welcome Keynote Address. 15th Biosimilar Medicines Conference: Biosimilar Medicines: A game changer for healthcare sustainability; 23−24 March 2017; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. European oncologists back biosimilars with position paper [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 17]. Available from: www.gabionline.net/Biosimilars/General/European-oncologists-back-biosimilars-with-position-paper
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment