An examination of biosimilar approvals by the US Food and Drug Administration (FDA) compared to launches was carried out by authors from Law360 [1].
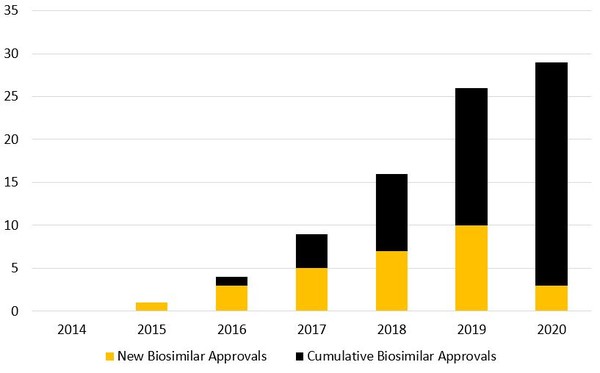
They found that biosimilar approvals have increased steadily from 2015‒2019, however, 2020 saw a huge reduction in approvals from a record 10 in 2019 to just three. The three biosimilars approved in 2020 were Pfizer’s Nyvepria (pegfilgrastim-apgf), Mylan’s Hulio (adalimumab-fkjp) and Amgen’s Riabni (rituximab-arrx) [2].
Although there were only three approvals in 2020, according to authors Whitehill and Deshmukh, there were five biosimilar launches. However, this was again a drop from 2019, when there were seven launches, see Figure 1. Launching in 2020 were Pfizer’s Ruxience (rituximab-pvvr), Amgen’s Avsola (infliximab-axxq) and three trastuzumab biosimilars: Pfizer’s Trazimera (trastuzumab-qyyp), Celltrion/Teva’s Herzuma (trastuzumab-pkrb) and Samsung Bioepis/Merck’s Ontruzant (trastuzumab-dttb).
Figure 1: Biosimilar approvals in the US
Source: Law360 Whitehall and Deshmukh [1].
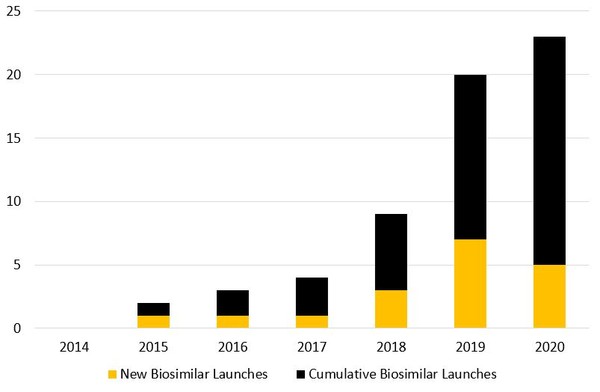
Of the 29 biosimilars approved in the US, only 18 have so far been launched in the country, see Figure 2. This number stands in stark contrast to Europe, where a whopping 69 biosimilars are approved for use, almost all of which are already marketed [3].
Figure 2: Biosimilar launches in the US
Source: Law360 Whitehall and Deshmukh [1].
Of the remaining 11, six are adalimumab biosimilars where the biosimilar companies have signed licensing agreements with AbbVie, who makes the adalimumab originator biological Humira. These agreements mean that these biosimilars (along with three other adalimumab biosimilars for which AbbVie has also made licensing agreements) cannot launch until 2023 [4]. This compares to 10 adalimumab biosimilars that have been approved and launched in Europe [3].
Two etanercept biosimilars (Erelzi and Etico) are also yet to launch due to being held up by patent litigation. Amgen was granted a new US patent on its blockbuster drug Enbrel (etanercept) in November 2011 that is expected to block biosimilars until 2028 [5].
One approved biosimilar that has not yet launched is a long-approved infliximab biosimilar, Ixifi, that Pfizer has said that it will not launch – the company has another infliximab biosimilar – Inflectra (infliximab-dyyb) already on the market.
The remaining two approved biosimilars that have yet to launch are Amgen’s Riabni (rituximab-arrx) and Pfizer’s Nyvepria (pegfilgrastim-apgf). Riabni was only approved in December 2020 and is expected to launch in January 2021, however, Pfizer’s Nyvepria is currently in litigation and therefore a launch date is not yet known.
Related articles
Biosimilar patent litigation trends in the US
Biosimilar approvals and patent litigation in the US
Advantages and challenges of biosimilars approved in Europe and the US
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. Whitehill J, Deshmukh J. After lull in biosimilar IP litigation, 2021 could bring influx. Law360. 2021 Jan 7.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Feb 26]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Feb 26]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
4. GaBI Online - Generics and Biosimilars Initiative. Boehringer Ingelheim finally signs licensing deal for Humira biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Feb 26]. Available from: www.gabionline.net/Pharma-News/Boehringer-Ingelheim-finally-signs-licensing-deal-for-Humira-biosimilar
5. GaBI Online - Generics and Biosimilars Initiative. New Amgen Enbrel patent could block biosimilars until 2028 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Feb 26]. Available from: www.gabionline.net/Biosimilars/News/New-Amgen-Enbrel-patent-could-block-biosimilars-until-2028
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Biosimilar patent litigation trends in the US








 0
0











Post your comment