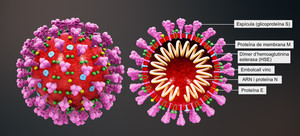
The global race for COVID-19 vaccines is on. New Zealand has allocated hundreds of millions of dollars in a bid to get quick access to a vaccine, once found. Although the actual sum has not yet been revealed, this is in addition to its NZ$37 million vaccination strategy.
The New Zealand government is already negotiating with pharmaceutical companies for access to vaccines that have not yet been fully developed and tested. However, some fear that those that develop the vaccine and control the patent may charge high prices and limit access.
So, if New Zealand’s government fails in its negotiations, it will have to look at its patent law to try to gain access to the drug product. When under a state of emergency, New Zealand law does allow compulsory licencing and use of patented products. However, under this law, such applications can only be made three years after failed negotiation with a patent holder. Contrary to this, in times of national emergency, international commercial law negates any need to negotiate with patent holders. An amendment to the current New Zealand law should be made that mirrors international law and includes a clear clause related to national emergencies. Currently, New Zealand and international law allow drugs manufactured under a compulsory licence to be exported to countries experiencing serious public health issues. This may be significant for the nations in the Pacific who are also in the grips of the pandemic.
Although the New Zealand government can gain access to patented inventions in times of emergency, drug products must continue to undergo full regulatory review. To gain a Medical Devices Safety Authority (Medsafe) approval a variety of information and data on safety, efficacy are required, and the process can be lengthy. This could delay access to potential vaccines in New Zealand and it has been suggested that if a vaccine has been approved in other regions, such as Australia, Canada or Europe, it should be able to gain automatic approval in New Zealand. In addition, under current legislation, generic versions of a COVID-19 vaccination could take five years to be approved. This is due to restrictions on the data used to approve generics, and it has been suggested that these be lifted to increase access to these vaccines by approving generic versions.
To ensure that New Zealand can gain quick access to a potential COVID-19 vaccine without hindrance, changes to its patent law are advised.
Related articles
Biocon and Celltrion make progress in the battle against COVID-19
Indian generics makers begin to dispatch remdesivir
COVID-19 drug trials underway
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.








 0
0









Post your comment