Biosimilars/Research
|
Posted 03/02/2017
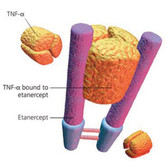
Several biologicals will lose patent protection within the next few years, opening up the market for biosimilars [1]. According to US Food and Drug Administration (FDA) guidelines, biosimilar applicants should demonstrate biosimilarity using a stepwise approach, which includes structural and functional characterization, animal toxicity, pharmacokinetics and pharmacodynamics, immunogenicity, and clinical safety and effectiveness [2]. FDA expects extensive characterization of the proposed biosimilar and the reference product using state-of-the-art analytical technology including analysis of the protein (primary, secondary, tertiary, quaternary structure as well as post-translational modifications).